Temporal Trends in SARS-CoV-2 Antibody Levels Among COVID-19 Patients in Kerala During the First Wave and Pre-vaccination Period
- PMID: 38966433
- PMCID: PMC11223736
- DOI: 10.7759/cureus.61650
Temporal Trends in SARS-CoV-2 Antibody Levels Among COVID-19 Patients in Kerala During the First Wave and Pre-vaccination Period
Abstract
Background: The SARS-CoV-2 virus interacts with host cells through the S1 domain of its spike protein. This study measures the IgG immune response to this domain in COVID-19 patients from Kerala, India, and explores its association with various health factors.
Methods: A cohort of 258 COVID-19 patients was analyzed for IgG antibodies targeting the S1 spike protein domain. The temporal pattern of the IgG response and its correlation with hospitalization needs, intensive care, and pre-existing conditions such as diabetes, hypertension, and coronary artery disease were assessed.
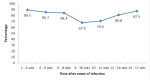
Results: A significant IgG response (76.4%) was detected, indicating robust immune activation post-infection. The IgG levels peaked between two to four and four to eight weeks post-infection, with a notable increase at 12 weeks, hinting at possible secondary exposure or an immune memory response. No correlation was found between IgG levels and the presence of diabetes mellitus, hypertension, or coronary artery disease. However, higher IgG responses correlated with the severity of the infection, as seen in patients requiring hospitalization or intensive care.
Conclusions: The IgG response to the S1 spike protein domain serves as a potential marker of immune activation in COVID-19. It reflects the body's defense mechanism against the virus and may predict disease severity and outcomes. The findings suggest that IgG levels could be indicative of the viral load, inflammatory response, and possibly the likelihood of protection against reinfection.
Keywords: antibody response; comorbidities; covid-19; covid-19 vaccination; first wave; igg; kerala; pre-vaccination; sars-cov-2; sars-cov-2 spike protein.
Copyright © 2024, Mathew et al.
Conflict of interest statement
Human subjects: Consent was obtained or waived by all participants in this study. Institutional Ethics Committee of Government Medical College, Kozhikode, issued approval GMCKKD/RP 2020/IEC/536 dated 18/NOV/2020. The study received ethical clearance from the Institutional Ethics Committee of Government Medical College, Kozhikode (Ref No. GMCKKD/RP 2020/IEC/536 dated 18/NOV/2020). Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue. Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work. Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work. Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Figures
References
-
- The humoral response and antibodies against SARS-CoV-2 infection. Qi H, Liu B, Wang X, Zhang L. Nat Immunol. 2022;23:1008–1020. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous