Rapid, Portable, and Electricity-free Sample Extraction Method for Enhanced Molecular Diagnostics in Resource-Limited Settings
- PMID: 38967089
- PMCID: PMC11256010
- DOI: 10.1021/acs.analchem.4c00319
Rapid, Portable, and Electricity-free Sample Extraction Method for Enhanced Molecular Diagnostics in Resource-Limited Settings
Abstract
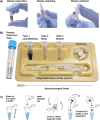
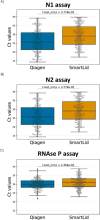
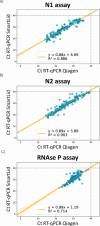
The COVID-19 pandemic has highlighted the need for rapid and reliable diagnostics that are accessible in resource-limited settings. To address this pressing issue, we have developed a rapid, portable, and electricity-free method for extracting nucleic acids from respiratory swabs (i.e. nasal, nasopharyngeal and buccal swabs), successfully demonstrating its effectiveness for the detection of SARS-CoV-2 in residual clinical specimens. Unlike traditional approaches, our solution eliminates the need for micropipettes or electrical equipment, making it user-friendly and requiring little to no training. Our method builds upon the principles of magnetic bead extraction and revolves around a low-cost plastic magnetic lid, called SmartLid, in combination with a simple disposable kit containing all required reagents conveniently prealiquoted. Here, we clinically validated the SmartLid sample preparation method in comparison to the gold standard QIAamp Viral RNA Mini Kit from QIAGEN, using 406 clinical isolates, including 161 SARS-CoV-2 positives, using the SARS-CoV-2 RT-qPCR assays developed by the US Centers for Disease Control and Prevention (CDC). The SmartLid method showed an overall sensitivity of 95.03% (95% CI: 90.44-97.83%) and a specificity of 99.59% (95% CI: 97.76-99.99%), with a positive agreement of 97.79% (95% CI: 95.84-98.98%) when compared to QIAGEN's column-based extraction method. There are clear benefits to using the SmartLid sample preparation kit: it enables swift extraction of viral nucleic acids, taking less than 5 min, without sacrificing significant accuracy when compared to more expensive and time-consuming alternatives currently available on the market. Moreover, its simplicity makes it particularly well-suited for the point-of-care where rapid results and portability are crucial. By providing an efficient and accessible means of nucleic acid extraction, our approach aims to introduce a step-change in diagnostic capabilities for resource-limited settings.
Conflict of interest statement
The authors declare the following competing financial interest(s): I.P., M.L.C., K.M.C., K.T.M., N.M., P.G. and J.R.M. have financial interest on ProtonDx Ltd, which currently has an exclusive license to the SmartLid intellectual property and trademark. All authors declare that they have no other conflict of interest related to this work. All the authors declare that they do not have any other known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
Similar articles
-
Highly sensitive extraction-free saliva-based molecular assay for rapid diagnosis of SARS-CoV-2.J Clin Microbiol. 2024 Jun 12;62(6):e0060024. doi: 10.1128/jcm.00600-24. Epub 2024 May 24. J Clin Microbiol. 2024. PMID: 38785448 Free PMC article.
-
Over 90% of clinical swabs used for SARS-CoV-2 diagnostics contain sufficient nucleic acid concentrations.J Med Virol. 2021 May;93(5):2848-2856. doi: 10.1002/jmv.26706. Epub 2021 Feb 16. J Med Virol. 2021. PMID: 33289117 Free PMC article.
-
Development and validation of a portable device for lab-free versatile nucleic acid extraction.Biotechniques. 2024 Oct;76(10):505-515. doi: 10.1080/07366205.2024.2427544. Epub 2024 Dec 2. Biotechniques. 2024. PMID: 39620898
-
Rapid, point-of-care antigen and molecular-based tests for diagnosis of SARS-CoV-2 infection.Cochrane Database Syst Rev. 2021 Mar 24;3(3):CD013705. doi: 10.1002/14651858.CD013705.pub2. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 Jul 22;7:CD013705. doi: 10.1002/14651858.CD013705.pub3. PMID: 33760236 Free PMC article. Updated.
-
The potential application of electrochemical biosensors in the COVID-19 pandemic: A perspective on the rapid diagnostics of SARS-CoV-2.Biosens Bioelectron. 2021 Mar 15;176:112905. doi: 10.1016/j.bios.2020.112905. Epub 2020 Dec 17. Biosens Bioelectron. 2021. PMID: 33358285 Free PMC article. Review.
Cited by
-
Democratization of Point-of-Care Viral Biosensors: Bridging the Gap from Academia to the Clinic.Biosensors (Basel). 2025 Jul 7;15(7):436. doi: 10.3390/bios15070436. Biosensors (Basel). 2025. PMID: 40710086 Free PMC article. Review.
-
Portable molecular diagnostic platform for rapid point-of-care detection of mpox and other diseases.Nat Commun. 2025 Mar 24;16(1):2875. doi: 10.1038/s41467-025-57647-3. Nat Commun. 2025. PMID: 40128193 Free PMC article.
References
-
- The academy of medical sciences . Antimicrobial Resistance Research: Learning Lessons from the COVID-19 Pandemic, 2021. https://acmedsci.ac.uk/file-download/73901389 (accessed Nov 12, 2023).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

