A Configurationally Stable Helical Indenofluorene
- PMID: 38967257
- PMCID: PMC11267600
- DOI: 10.1021/acs.orglett.4c02128
A Configurationally Stable Helical Indenofluorene
Abstract
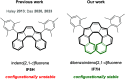
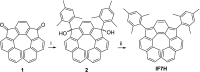
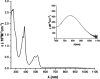
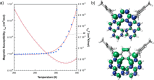
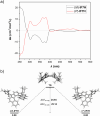
We report the synthesis and study of the optoelectronic, magnetic, and chiroptical properties of a helically chiral diradicaloid based on dibenzoindeno[2,1-c]fluorene. The molecule shows a small HOMO-LUMO gap and a moderate singlet-triplet gap, which agrees with the results of DFT calculations. The helical structure of the compound, confirmed by X-ray diffraction, is configurationally stable, which allows the isolation of both enantiomers and the evaluation of the chiroptical properties (ECD).
Conflict of interest statement
The authors declare no competing financial interest.
Figures

References
-
- Can A.; Facchetti A.; Usta H. Indenofluorenes for Organic Optoelectronics: the Dance of Fused Five- and Six-Membered Rings Enabling Structural Versatility. J. Mater. Chem. C 2022, 10, 8496–8535. 10.1039/D2TC00684G. - DOI
-
- Dressler J. J.; Zhou Z.; Marshall J. L.; Kishi R.; Takamuku S.; Wei Z.; Spisak S. N.; Nakano M.; Petrukhina M. A.; Haley M. M. Synthesis of the Unknown Indeno[1,2-a]fluorene Regioisomer: Crystallographic Characterization of Its Dianion. Angew. Chem., Int. Ed. 2017, 56, 15363–15367. 10.1002/anie.201709282. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

