Loss of β-catenin reveals a role for glutathione in regulating oxidative stress during cholestatic liver disease
- PMID: 38967587
- PMCID: PMC11227358
- DOI: 10.1097/HC9.0000000000000485
Loss of β-catenin reveals a role for glutathione in regulating oxidative stress during cholestatic liver disease
Abstract
Background: Cholestasis is an intractable liver disorder that results from impaired bile flow. We have previously shown that the Wnt/β-catenin signaling pathway regulates the progression of cholestatic liver disease through multiple mechanisms, including bile acid metabolism and hepatocyte proliferation. To further explore the impact of these functions during intrahepatic cholestasis, we exposed mice to a xenobiotic that causes selective biliary injury.
Methods: α-naphthylisothiocyanate (ANIT) was administered to liver-specific knockout (KO) of β-catenin and wild-type mice in the diet. Mice were killed at 6 or 14 days to assess the severity of cholestatic liver disease, measure the expression of target genes, and perform biochemical analyses.
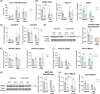
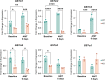
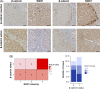
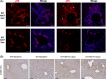
Results: We found that the presence of β-catenin was protective against ANIT, as KO mice had a significantly lower survival rate than wild-type mice. Although serum markers of liver damage and total bile acid levels were similar between KO and wild-type mice, the KO had minor histological abnormalities, such as sinusoidal dilatation, concentric fibrosis around ducts, and decreased inflammation. Notably, both total glutathione levels and expression of glutathione-S-transferases, which catalyze the conjugation of ANIT to glutathione, were significantly decreased in KO after ANIT. Nuclear factor erythroid-derived 2-like 2, a master regulator of the antioxidant response, was activated in KO after ANIT as well as in a subset of patients with primary sclerosing cholangitis lacking activated β-catenin. Despite the activation of nuclear factor erythroid-derived 2-like 2, KO livers had increased lipid peroxidation and cell death, which likely contributed to mortality.
Conclusions: Loss of β-catenin leads to increased cellular injury and cell death during cholestasis through failure to neutralize oxidative stress, which may contribute to the pathology of this disease.
Copyright © 2024 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Association for the Study of Liver Diseases.
Conflict of interest statement
Satdarshan P. Monga consults for Alnylam, AntlerA, Mermaid Bio, Surrozen, UbiquiTx, and Vicero. He received grants from Alnylam and Fog. Kari Nejak-Bowen consults for Surrozen. The remaining authors have no conflicts to report.
Figures




References
-
- Lazaridis KN, Strazzabosco M, Larusso NF. The cholangiopathies: Disorders of biliary epithelia. Gastroenterology. 2004;127:1565–77. - PubMed
-
- Dietrich CG, Ottenhoff R, de Waart DR, Oude Elferink RP. Role of MRP2 and GSH in intrahepatic cycling of toxins. Toxicology. 2001;167:73–81. - PubMed
-
- Connolly AK, Price SC, Connelly JC, Hinton RH. Early changes in bile duct lining cells and hepatocytes in rats treated with alpha-naphthylisothiocyanate. Toxicol Appl Pharmacol. 1988;93:208–19. - PubMed
-
- Copple BL, Jaeschke H, Klaassen CD. Oxidative stress and the pathogenesis of cholestasis. Semin Liver Dis. 2010;30:195–204. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

