Gut microbial metabolites in MASLD: Implications of mitochondrial dysfunction in the pathogenesis and treatment
- PMID: 38967596
- PMCID: PMC11227362
- DOI: 10.1097/HC9.0000000000000484
Gut microbial metabolites in MASLD: Implications of mitochondrial dysfunction in the pathogenesis and treatment
Abstract
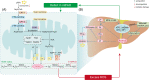
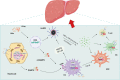
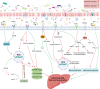
With an increasing prevalence, metabolic dysfunction-associated steatotic liver disease (MASLD) has become a major global health problem. MASLD is well-known as a multifactorial disease. Mitochondrial dysfunction and alterations in the gut bacteria are 2 vital events in MASLD. Recent studies have highlighted the cross-talk between microbiota and mitochondria, and mitochondria are recognized as pivotal targets of the gut microbiota to modulate the host's physiological state. Mitochondrial dysfunction plays a vital role in MASLD and is associated with multiple pathological changes, including hepatocyte steatosis, oxidative stress, inflammation, and fibrosis. Metabolites are crucial mediators of the gut microbiota that influence extraintestinal organs. Additionally, regulation of the composition of gut bacteria may serve as a promising therapeutic strategy for MASLD. This study reviewed the potential roles of several common metabolites in MASLD, emphasizing their impact on mitochondrial function. Finally, we discuss the current treatments for MASLD, including probiotics, prebiotics, antibiotics, and fecal microbiota transplantation. These methods concentrate on restoring the gut microbiota to promote host health.
Copyright © 2024 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Association for the Study of Liver Diseases.
Conflict of interest statement
The authors have no conflicts to report.
Figures

Similar articles
-
Microbiome-centered therapies for the management of metabolic dysfunction-associated steatotic liver disease.Clin Mol Hepatol. 2025 Feb;31(Suppl):S94-S111. doi: 10.3350/cmh.2024.0811. Epub 2024 Nov 28. Clin Mol Hepatol. 2025. PMID: 39604327 Free PMC article. Review.
-
Metabolic dysfunction-associated steatotic liver disease and the gut microbiome: pathogenic insights and therapeutic innovations.J Clin Invest. 2025 Apr 1;135(7):e186423. doi: 10.1172/JCI186423. J Clin Invest. 2025. PMID: 40166938 Free PMC article. Review.
-
Advances in the acting mechanism and treatment of gut microbiota in metabolic dysfunction-associated steatotic liver disease.Gut Microbes. 2025 Dec;17(1):2500099. doi: 10.1080/19490976.2025.2500099. Epub 2025 May 20. Gut Microbes. 2025. PMID: 40394806 Free PMC article. Review.
-
The Microbiome and Metabolic Dysfunction-Associated Steatotic Liver Disease.Int J Mol Sci. 2025 Mar 22;26(7):2882. doi: 10.3390/ijms26072882. Int J Mol Sci. 2025. PMID: 40243472 Free PMC article. Review.
-
Dietary Strategies to Modulate Gut Microbiota in Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD).Nutrients. 2025 Jun 1;17(11):1906. doi: 10.3390/nu17111906. Nutrients. 2025. PMID: 40507175 Free PMC article. Review.
Cited by
-
Biomarkers for Health Functional Foods in Metabolic Dysfunction-Associated Steatotic Liver Disorder (MASLD) Prevention: An Integrative Analysis of Network Pharmacology, Gut Microbiota, and Multi-Omics.Nutrients. 2024 Sep 11;16(18):3061. doi: 10.3390/nu16183061. Nutrients. 2024. PMID: 39339660 Free PMC article. Review.
-
Thermogenesis and Energy Metabolism in Brown Adipose Tissue in Animals Experiencing Cold Stress.Int J Mol Sci. 2025 Mar 31;26(7):3233. doi: 10.3390/ijms26073233. Int J Mol Sci. 2025. PMID: 40244078 Free PMC article. Review.
-
Altered mitochondrial function: a clue therapeutic strategies between metabolic dysfunction-associated steatotic liver disease and chronic kidney disease?Front Nutr. 2025 Jun 13;12:1613640. doi: 10.3389/fnut.2025.1613640. eCollection 2025. Front Nutr. 2025. PMID: 40584092 Free PMC article. Review.
-
The metabolites of gut microbiota: their role in ferroptosis in inflammatory bowel disease.Eur J Med Res. 2025 Apr 7;30(1):248. doi: 10.1186/s40001-025-02524-4. Eur J Med Res. 2025. PMID: 40189555 Free PMC article. Review.
-
MASLD: Prevalence, Mechanisms, and Sex-Based Therapies in Postmenopausal Women.Biomedicines. 2025 Apr 2;13(4):855. doi: 10.3390/biomedicines13040855. Biomedicines. 2025. PMID: 40299427 Free PMC article. Review.
References
-
- Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. . The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328–357. - PubMed
-
- Malhi H, Brown RS, Jr, Lim JK, Reau N, Tapper EB, Wong CC, et al. . Precipitous changes in nomenclature and definitions-NAFLD becomes SLD: Implications for and expectations of AASLD journals. Hepatology. 2023;78:1680–1681. - PubMed
-
- Wu Y, Zheng Q, Zou B, Yeo YH, Li X, Li J, et al. . The epidemiology of NAFLD in Mainland China with analysis by adjusted gross regional domestic product: A meta-analysis. Hepatol Int. 2020;14:259–269. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

