Technology advances in diabetes pregnancy: right technology, right person, right time
- PMID: 38967667
- PMCID: PMC11447080
- DOI: 10.1007/s00125-024-06216-2
Technology advances in diabetes pregnancy: right technology, right person, right time
Abstract
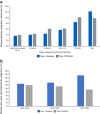
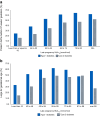
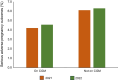
This review outlines some of the extraordinary recent advances in diabetes technology, which are transforming the management of type 1 diabetes before, during and after pregnancy. It highlights recent improvements associated with use of continuous glucose monitoring (CGM) but acknowledges that neither CGM nor insulin pump therapy are adequate for achieving the pregnancy glucose targets. Furthermore, even hybrid closed-loop (HCL) systems that are clinically effective outside of pregnancy may not confer additional benefits throughout pregnancy. To date, there is only one HCL system, the CamAPS FX, with a strong evidence base for use during pregnancy, suggesting that the pregnancy benefits are HCL system specific. This is in stark contrast to HCL system use outside of pregnancy, where benefits are HCL category specific. The CamAPS FX HCL system has a rapidly adaptive algorithm and lower glucose targets with benefits across all maternal glucose categories, meaning that it is applicable for all women with type 1 diabetes, before and during pregnancy. For women of reproductive years living with type 2 diabetes, the relative merits of using non-insulin pharmacotherapies vs diabetes technology (dipeptidyl peptidase-4 inhibitors, glucagon-like peptide-1 receptor agonists and sodium-glucose cotransporter 2 inhibitors) are unknown. Despite the urgent unmet need and potential benefits, studies of pharmacotherapy and technology use are extremely limited in pregnant women with type 2 diabetes.
Keywords: Automated insulin delivery; Closed-Loop; Continuous glucose monitoring; Diabetes technology; Neonatal; Obstetric; Pregnancy; Review; Type 1 Diabetes; Type 2 Diabetes.
© 2024. The Author(s).
Figures
References
-
- Murphy HR, Howgate C, O’Keefe J et al (2021) Characteristics and outcomes of pregnant women with type 1 or type 2 diabetes: a 5-year national population-based cohort study. Lancet Diabetes Endocrinol 9(3):153–164. 10.1016/S2213-8587(20)30406-X - PubMed
-
- National Pregnancy in Diabetes (NPID) Audit Report 2020. https://digital.nhs.uk/data-and-information/publications/statistical/nat... United Kingdom. Last accessed 01 May 2024
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

