tRNA-derived fragments in T lymphocyte-beta cell crosstalk and in type 1 diabetes pathogenesis in NOD mice
- PMID: 38967669
- PMCID: PMC11446995
- DOI: 10.1007/s00125-024-06207-3
tRNA-derived fragments in T lymphocyte-beta cell crosstalk and in type 1 diabetes pathogenesis in NOD mice
Abstract
Aims/hypothesis: tRNAs play a central role in protein synthesis. Besides this canonical function, they were recently found to generate non-coding RNA fragments (tRFs) regulating different cellular activities. The aim of this study was to assess the involvement of tRFs in the crosstalk between immune cells and beta cells and to investigate their contribution to the development of type 1 diabetes.
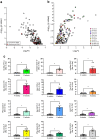
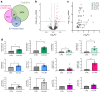
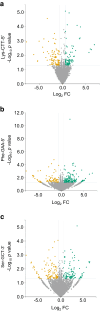
Methods: Global profiling of the tRFs present in pancreatic islets of 4- and 8-week-old NOD mice and in extracellular vesicles released by activated CD4+ T lymphocytes was performed by small RNA-seq. Changes in the level of specific fragments were confirmed by quantitative PCR. The transfer of tRFs from immune cells to beta cells occurring during insulitis was assessed using an RNA-tagging approach. The functional role of tRFs increasing in beta cells during the initial phases of type 1 diabetes was determined by overexpressing them in dissociated islet cells and by determining the impact on gene expression and beta cell apoptosis.
Results: We found that the tRF pool was altered in the islets of NOD mice during the initial phases of type 1 diabetes. Part of these changes were triggered by prolonged exposure of beta cells to proinflammatory cytokines (IL-1β, TNF-α and IFN-γ) while others resulted from the delivery of tRFs produced by CD4+ T lymphocytes infiltrating the islets. Indeed, we identified several tRFs that were enriched in extracellular vesicles from CD4+/CD25- T cells and were transferred to beta cells upon adoptive transfer of these immune cells in NOD.SCID mice. The tRFs delivered to beta cells during the autoimmune reaction triggered gene expression changes that affected the immune regulatory capacity of insulin-secreting cells and rendered the cells more prone to apoptosis.
Conclusions/interpretation: Our data point to tRFs as novel players in the crosstalk between the immune system and insulin-secreting cells and suggest a potential involvement of this novel class of non-coding RNAs in type 1 diabetes pathogenesis.
Data availability: Sequences are available from the Gene Expression Omnibus (GEO) with accession numbers GSE242568 and GSE256343.
Keywords: Apoptosis; Autoimmunity; Extracellular vesicles; Insulin; Pancreatic islet.
© 2024. The Author(s).
Figures




References
-
- Quattrin T, Mastrandrea LD, Walker LSK (2023) Type 1 diabetes. Lancet 401(10394):2149–2162. 10.1016/S0140-6736(23)00223-4 - PubMed
-
- van Belle TL, Coppieters KT, von Herrath MG (2011) Type 1 diabetes: etiology, immunology, and therapeutic strategies. Physiol Rev 91(1):79–118. 10.1152/physrev.00003.2010 - PubMed
-
- Eizirik DL, Colli ML, Ortis F (2009) The role of inflammation in insulitis and beta-cell loss in type 1 diabetes. Nat Rev Endocrinol 5(4):219–226. 10.1038/nrendo.2009.21 - PubMed
-
- Guay C, Kruit JK, Rome S et al (2019) Lymphocyte-derived exosomal microRNAs promote pancreatic beta cell death and may contribute to type 1 diabetes development. Cell Metab 29(2):348–361, e346. 10.1016/j.cmet.2018.09.011 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

