A randomized phase III double-blind placebo-controlled trial of first-line chemotherapy and trastuzumab with or without bevacizumab for patients with HER2/neu-positive metastatic breast cancer: a trial of the ECOG-ACRIN Cancer Research Group (E1105)
- PMID: 38967884
- PMCID: PMC11297090
- DOI: 10.1007/s10549-024-07417-4
A randomized phase III double-blind placebo-controlled trial of first-line chemotherapy and trastuzumab with or without bevacizumab for patients with HER2/neu-positive metastatic breast cancer: a trial of the ECOG-ACRIN Cancer Research Group (E1105)
Abstract
Background: In 2008, bevacizumab received accelerated Food and Drug Administration (FDA) approval for use in human epidermal growth factor receptor 2 (HER2)-negative metastatic breast cancer (MBC). Based on the pre-clinical and preliminary clinical activity of the trastuzumab and bevacizumab combination, ECOG-ACRIN E1105 trial was developed to determine if the addition of bevacizumab to a chemotherapy and trastuzumab combination for first-line therapy would improve progression-free survival (PFS) in patients with HER2-positive MBC.
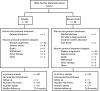
Findings: 96 patients were randomized to receive standard first-line chemotherapy and trastuzumab with or without bevacizumab between November 2007 and October 2009, and 93 began protocol therapy. Induction therapy was given for 24 weeks, followed by maintenance trastuzumab with or without bevacizumab. 60% (56/93) began carboplatin and 74% (69/93) completed 6 cycles of induction therapy. Primary endpoint was PFS. Median PFS was 11.1 and 13.8 months for placebo and bevacizumab arms, respectively (hazard ratio [HR] 95%, Confidence Interval [Cl] for bevacizumab vs. placebo: 0.73 [0.43-1.23], p = 0.24), and at a median follow-up of 70.7 months, median survival was 49.1 and 63 months (HR [95% Cl] for OS: 1.09 [0.61-1.97], p = 0.75). The most common toxicities across both arms were neutropenia and hypertension, with left ventricular systolic dysfunction, fatigue, and sensory neuropathy reported more frequently with bevacizumab.
Conclusions: In this trial, the addition of bevacizumab did not improve outcomes in patients with metastatic HER2-positive breast cancer. Although the trial was underpowered due to smaller than anticipated sample size, these findings corroborated other clinical trials during this time.
Clinical trial information: NCT00520975.
Keywords: Bevacizumab; Breast cancer; Chemotherapy; Trastuzumab.
© 2024. The Author(s).
Conflict of interest statement
JMS, AON, TJS, WJG, EAP, and ACW have no conflicts of interest related to this project. CLA has served as scientific advisor to Novartis, Lilly, Merck, Immunomedics, Daiichi Sankyo, AstraZeneca, PUMA Biotechnology, TAIHO Oncology, OrigiMed, Arvinas, Sanofi, and the Susan G. Komen Foundation. He has received grant support from Pfizer, Lilly, and Takeda. NJM is an employee of Flatiron Health, Inc. an independent member of the Roche group, and reports stock ownership in Roche. VGA has served as a scientific advisor for GuardantHealth and Astra Zeneca and has research funding support from Genetech, Bayer, and GuardantHealth. NUL declares consulting honorarium from Seagen, Daichii-Sankyo, AstraZeneca, Olema Pharmaceuticals, Janssen, Blueprint Medicines, Stemline/Menarini, Artera Inc., Eisai; travel support from Olema Pharmaceuticals; research support (to institution) from Genentech, Zion Pharmaceuticals (as part of GNE), Pfizer, Seagen, Merck, Olema Pharmaceuticals, AstraZeneca; royalties from Up To Date. SAR has served as a consultant for Daiichi-Sankyo, Astra Zeneca, Novartis, and Gilead.
Figures
Update of
-
A randomized phase III double-blind placebo-controlled trial of first line chemotherapy and trastuzumab with or without bevacizumab for patients with HER2/neu-positive metastatic breast cancer: a trial of the ECOG-ACRIN Cancer Research Group (E1105).Res Sq [Preprint]. 2024 Apr 29:rs.3.rs-4295044. doi: 10.21203/rs.3.rs-4295044/v1. Res Sq. 2024. Update in: Breast Cancer Res Treat. 2024 Sep;207(2):275-282. doi: 10.1007/s10549-024-07417-4. PMID: 38746356 Free PMC article. Updated. Preprint.
References
-
- Linderholm B, Andersson J, Lindh B, Beckman L, Erlanson M, Edin K et al (2004) Overexpression of c-erbB-2 is related to a higher expression of vascular endothelial growth factor (VEGF) and constitutes an independent prognostic factor in primary node-positive breast cancer after adjuvant systemic treatment. Eur J Cancer 40(1):33–42 10.1016/S0959-8049(03)00673-7 - DOI - PubMed
-
- Petit AM, Rak J, Hung MC, Rockwell P, Goldstein N, Fendly B et al (1997) Neutralizing antibodies against epidermal growth factor and ErbB-2/neu receptor tyrosine kinases down-regulate vascular endothelial growth factor production by tumor cells in vitro and in vivo: angiogenic implications for signal transduction therapy of solid tumors. Am J Pathol 151(6):1523–1530 - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- U10CA180888/CA/NCI NIH HHS/United States
- UG1 CA233270/CA/NCI NIH HHS/United States
- UG1CA233270/CA/NCI NIH HHS/United States
- UG1 CA233320/CA/NCI NIH HHS/United States
- UG1CA233234/RC/CCR NIH HHS/United States
- UG1CA189830/CA/NCI NIH HHS/United States
- UG1CA190140/CA/NCI NIH HHS/United States
- U24 CA196172/CA/NCI NIH HHS/United States
- UG1CA233196/CA/NCI NIH HHS/United States
- UG1CA233302/CA/NCI NIH HHS/United States
- UG1 CA233302/CA/NCI NIH HHS/United States
- UG1 CA233196/CA/NCI NIH HHS/United States
- U10 CA180820/CA/NCI NIH HHS/United States
- U10 CA180888/CA/NCI NIH HHS/United States
- UG1CA189828/CA/NCI NIH HHS/United States
- UG1CA233320/CA/NCI NIH HHS/United States
- UG1 CA190140/CA/NCI NIH HHS/United States
- UG1 CA233234/CA/NCI NIH HHS/United States
- UG1CA189805/CA/NCI NIH HHS/United States
- U10CA180821/CA/NCI NIH HHS/United States
- U10 CA180821/CA/NCI NIH HHS/United States
- UG1 CA189805/CA/NCI NIH HHS/United States
- UG1CA189954/CA/NCI NIH HHS/United States
- UG1 CA189828/CA/NCI NIH HHS/United States
- UG1 CA189830/CA/NCI NIH HHS/United States
- UG1 CA189954/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous