Mixture and Non-mixture Cure Models for Health Technology Assessment: What You Need to Know
- PMID: 38967908
- PMCID: PMC11405446
- DOI: 10.1007/s40273-024-01406-7
Mixture and Non-mixture Cure Models for Health Technology Assessment: What You Need to Know
Abstract
There is increasing interest in the use of cure modelling to inform health technology assessment (HTA) due to the development of new treatments that appear to offer the potential for cure in some patients. However, cure models are often not included in evidence dossiers submitted to HTA agencies, and they are relatively rarely relied upon to inform decision-making. This is likely due to a lack of understanding of how cure models work, what they assume, and how reliable they are. In this tutorial we explain why and when cure models may be useful for HTA, describe the key characteristics of mixture and non-mixture cure models, and demonstrate their use in a range of scenarios, providing Stata code. We highlight key issues that must be taken into account by analysts when fitting these models and by reviewers and decision-makers when interpreting their predictions. In particular, we note that flexible parametric non-mixture cure models have not been used in HTA, but they offer advantages that make them well suited to an HTA context when a cure assumption is valid but follow-up is limited.
© 2024. The Author(s).
Conflict of interest statement
N.R.L.'s institutions have received consultancy fees and funding, unrelated to the submitted work, from BMS, Merck, Sharpe and Dohme, and from Daiichi Sankyo, GSK, Janssen, Amgen, Sanofi, for projects N.R.L. has worked on.
Figures

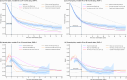
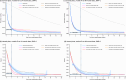

Similar articles
-
Avoiding and identifying errors in health technology assessment models: qualitative study and methodological review.Health Technol Assess. 2010 May;14(25):iii-iv, ix-xii, 1-107. doi: 10.3310/hta14250. Health Technol Assess. 2010. PMID: 20501062 Review.
-
Appraisals by Health Technology Assessment Agencies of Economic Evaluations Submitted as Part of Reimbursement Dossiers for Oncology Treatments: Evidence from Canada, the UK, and Australia.Curr Oncol. 2022 Oct 13;29(10):7624-7636. doi: 10.3390/curroncol29100602. Curr Oncol. 2022. PMID: 36290879 Free PMC article.
-
Resource modelling: the missing piece of the HTA jigsaw?Pharmacoeconomics. 2015 Mar;33(3):193-203. doi: 10.1007/s40273-014-0228-9. Pharmacoeconomics. 2015. PMID: 25411095
-
Health technology assessment of medical devices: What is different? An overview of three European projects.Z Evid Fortbild Qual Gesundhwes. 2015;109(4-5):309-18. doi: 10.1016/j.zefq.2015.06.011. Epub 2015 Jul 26. Z Evid Fortbild Qual Gesundhwes. 2015. PMID: 26354131 Review.
-
Health technology assessment of medical devices: a survey of non-European union agencies.Int J Technol Assess Health Care. 2015 Jan;31(3):154-65. doi: 10.1017/S0266462315000185. Epub 2015 Jun 5. Int J Technol Assess Health Care. 2015. PMID: 26044729 Free PMC article.
Cited by
-
Evaluating the Role and Policy Implications of Using External Evidence in Survival Extrapolations: A Case Study of Axicabtagene Ciloleucel Therapy for Second-Line DLBCL.Pharmacoeconomics. 2025 Aug 7. doi: 10.1007/s40273-025-01529-5. Online ahead of print. Pharmacoeconomics. 2025. PMID: 40775201
-
Cost-Effectiveness of Pembrolizumab Monotherapy for High Programmed Death Ligand 1 Advanced or Metastatic Non-small Cell Lung Cancer Depends on Long-Term Survivors.Clin Drug Investig. 2025 Aug;45(8):583-598. doi: 10.1007/s40261-025-01456-5. Epub 2025 Jul 9. Clin Drug Investig. 2025. PMID: 40632461 Free PMC article.
-
An Evaluation of an Algorithm for the Selection of Flexible Survival Models for Cancer Immunotherapies: Pass or Fail?Pharmacoeconomics. 2024 Dec;42(12):1395-1412. doi: 10.1007/s40273-024-01429-0. Epub 2024 Sep 20. Pharmacoeconomics. 2024. PMID: 39302594 Free PMC article.
-
A Multistate Model Incorporating Relative Survival Extrapolation and Mixed Time Scales for Health Technology Assessment.Pharmacoeconomics. 2025 Mar;43(3):297-310. doi: 10.1007/s40273-024-01457-w. Epub 2024 Nov 25. Pharmacoeconomics. 2025. PMID: 39586963 Free PMC article.
References
-
- National Institute for Health and Care Excellence. NICE health technology evaluations: the manual. NICE; 2022.
-
- Latimer N. NICE DSU Technical Support Document 14: undertaking survival analysis for economic evaluation alongside clinical trials - Extrapolation with patient-level data. 2011, NICE Decision Support Unit. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

