Probiotics and Antibiotic-Induced Microbial Aberrations in Children: A Secondary Analysis of a Randomized Clinical Trial
- PMID: 38967929
- PMCID: PMC11227081
- DOI: 10.1001/jamanetworkopen.2024.18129
Probiotics and Antibiotic-Induced Microbial Aberrations in Children: A Secondary Analysis of a Randomized Clinical Trial
Abstract
Importance: Probiotics are often considered in children to prevent antibiotic-associated diarrhea. However, the underlying mechanistic effects and impact of probiotics on antibiotic-induced microbiota changes are not well understood.
Objective: To investigate the effects of a multispecies probiotic on the gut microbiota composition in children receiving antibiotics.
Design, setting, and participants: This is a secondary analysis of a randomized, quadruple-blind, placebo-controlled clinical trial from February 1, 2018, to May 31, 2021, including 350 children receiving broad-spectrum antibiotics in the inpatient and outpatient settings. Patients were followed up until 1 month after the intervention period. Fecal samples and data were analyzed between September 1, 2022, and February 28, 2023. Eligibility criteria included 3 months to 18 years of age and recruitment within 24 hours following initiation of broad-spectrum systemic antibiotics. In total, 646 eligible patients were approached and 350 participated in the trial.
Intervention: Participants were randomly assigned to receive daily placebo or a multispecies probiotic formulation consisting of 8 strains from 5 different genera during antibiotic treatment and for 7 days afterward.
Main outcomes and measures: Fecal stool samples were collected at 4 predefined times: (1) inclusion, (2) last day of antibiotic use, (3) last day of the study intervention, and (4) 1 month after intervention. Microbiota analysis was performed by 16S ribosomal RNA gene sequencing.
Results: A total of 350 children were randomized and collected stool samples from 88 were eligible for the microbiota analysis (54 boys and 34 girls; mean [SD] age, 47.09 [55.64] months). Alpha diversity did not significantly differ between groups at the first 3 times. Shannon diversity (mean [SD], 3.56 [0.75] vs 3.09 [1.00]; P = .02) and inverse Simpson diversity (mean [SD], 3.75 [95% CI, 1.66-5.82] vs -1.31 [95% CI, -3.17 to 0.53]; P = 1 × 10-4) indices were higher in the placebo group compared with the probiotic group 1 month after intervention. Beta diversity was not significantly different at any of the times. Three of 5 supplemented genera had higher relative abundance during probiotic supplementation, but this difference had disappeared after 1 month.
Conclusions and relevance: The studied probiotic mixture had minor and transient effects on the microbiota composition during and after antibiotic treatment. Further research is needed to understand their working mechanisms in manipulating the microbiome and preventing antibiotic-associated dysbiosis and adverse effects such as antibiotic-associated diarrhea.
Trial registration: ClinicalTrials.gov Identifier: NCT03334604.
Conflict of interest statement
Figures
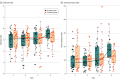


References
-
- Allwell-Brown G, Hussain-Alkhateeb L, Kitutu FE, Strömdahl S, Mårtensson A, Johansson EW. Trends in reported antibiotic use among children under 5 years of age with fever, diarrhoea, or cough with fast or difficult breathing across low-income and middle-income countries in 2005-17: a systematic analysis of 132 national surveys from 73 countries. Lancet Glob Health. 2020;8(6):e799-e807. doi:10.1016/S2214-109X(20)30079-6 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

