Protocol for adeno-associated virus-mediated optogenetic activation of olfactory output neurons in neonatal mice
- PMID: 38968078
- PMCID: PMC11452914
- DOI: 10.1016/j.xpro.2024.103164
Protocol for adeno-associated virus-mediated optogenetic activation of olfactory output neurons in neonatal mice
Abstract
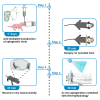
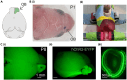
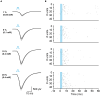
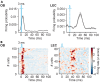
Optogenetic manipulation has proven a powerful tool for investigating the mechanisms underlying the function of neuronal networks, but implementing the technique on mammals during early development remains challenging. Here, we present a comprehensive workflow to specifically manipulate mitral/tufted cells (M/TCs), the output neurons in the olfactory circuit, mediated by adeno-associated virus (AAV) transduction and light stimulation in neonatal mice and monitor neuronal and network activity with in vivo electrophysiology. This method represents an efficient approach to elucidate functional brain development. For complete details on the use and execution of this protocol, please refer to Chen et al.1,2,3.
Keywords: biophysics; developmental biology; neuroscience; systems biology.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures



Similar articles
-
Cell type-specific and frequency-dependent centrifugal modulation in olfactory bulb output neurons in vivo.J Neurophysiol. 2024 Jun 1;131(6):1226-1239. doi: 10.1152/jn.00078.2024. Epub 2024 May 1. J Neurophysiol. 2024. PMID: 38691531 Free PMC article.
-
CCKergic Tufted Cells Differentially Drive Two Anatomically Segregated Inhibitory Circuits in the Mouse Olfactory Bulb.J Neurosci. 2020 Aug 5;40(32):6189-6206. doi: 10.1523/JNEUROSCI.0769-20.2020. Epub 2020 Jun 30. J Neurosci. 2020. PMID: 32605937 Free PMC article.
-
The Interglomerular Circuit Potently Inhibits Olfactory Bulb Output Neurons by Both Direct and Indirect Pathways.J Neurosci. 2016 Sep 14;36(37):9604-17. doi: 10.1523/JNEUROSCI.1763-16.2016. J Neurosci. 2016. PMID: 27629712 Free PMC article.
-
Intersectional targeting of defined neural circuits by adeno-associated virus vectors.J Neurosci Res. 2021 Apr;99(4):981-990. doi: 10.1002/jnr.24774. Epub 2020 Dec 20. J Neurosci Res. 2021. PMID: 33341969 Free PMC article. Review.
-
Subpopulations of Projection Neurons in the Olfactory Bulb.Front Neural Circuits. 2020 Aug 28;14:561822. doi: 10.3389/fncir.2020.561822. eCollection 2020. Front Neural Circuits. 2020. PMID: 32982699 Free PMC article. Review.
References
-
- Gretenkord S., Kostka J.K., Hartung H., Watznauer K., Fleck D., Minier-Toribio A., Spehr M., Hanganu-Opatz I.L. Coordinated electrical activity in the olfactory bulb gates the oscillatory entrainment of entorhinal networks in neonatal mice. PLoS Biol. 2019;17 doi: 10.1371/journal.pbio.2006994. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

