Flp/ FRT-mediated disruption of ptex150 and exp2 in Plasmodium falciparum sporozoites inhibits liver-stage development
- PMID: 38968107
- PMCID: PMC11252984
- DOI: 10.1073/pnas.2403442121
Flp/ FRT-mediated disruption of ptex150 and exp2 in Plasmodium falciparum sporozoites inhibits liver-stage development
Abstract
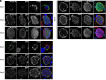
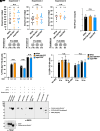
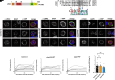
Plasmodium falciparum causes severe malaria and assembles a protein translocon (PTEX) complex at the parasitophorous vacuole membrane (PVM) of infected erythrocytes, through which several hundred proteins are exported to facilitate growth. The preceding liver stage of infection involves growth in a hepatocyte-derived PVM; however, the importance of protein export during P. falciparum liver infection remains unexplored. Here, we use the FlpL/FRT system to conditionally excise genes in P. falciparum sporozoites for functional liver-stage studies. Disruption of PTEX members ptex150 and exp2 did not affect sporozoite development in mosquitoes or infectivity for hepatocytes but attenuated liver-stage growth in humanized mice. While PTEX150 deficiency reduced fitness on day 6 postinfection by 40%, EXP2 deficiency caused 100% loss of liver parasites, demonstrating that PTEX components are required for growth in hepatocytes to differing degrees. To characterize PTEX loss-of-function mutations, we localized four liver-stage Plasmodium export element (PEXEL) proteins. P. falciparum liver specific protein 2 (LISP2), liver-stage antigen 3 (LSA3), circumsporozoite protein (CSP), and a Plasmodium berghei LISP2 reporter all localized to the periphery of P. falciparum liver stages but were not exported beyond the PVM. Expression of LISP2 and CSP but not LSA3 was reduced in ptex150-FRT and exp2-FRT liver stages, suggesting that expression of some PEXEL proteins is affected directly or indirectly by PTEX disruption. These results show that PTEX150 and EXP2 are important for P. falciparum development in hepatocytes and emphasize the emerging complexity of PEXEL protein trafficking.
Keywords: hepatocyte; humanized mice; malaria; protein export; translocon.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures



References
-
- WHO, World malaria report 2023 (Geneva, 2023). https://www.who.int/teams/global-malaria-programme/reports/world-malaria.... Accessed 1 February 2024.
-
- de Koning-Ward T. F., Dixon M. W., Tilley L., Gilson P. R., Plasmodium species: Master renovators of their host cells. Nat. Rev. Microbiol. 14, 494–507 (2016). - PubMed
-
- Marti M., Good R. T., Rug M., Knuepfer E., Cowman A. F., Targeting malaria virulence and remodeling proteins to the host erythrocyte. Science 306, 1930–1933 (2004). - PubMed
-
- Hiller N. L., et al. , A host-targeting signal in virulence proteins reveals a secretome in malarial infection. Science 306, 1934–1937 (2004). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

