Selective Btk inhibition by PRN1008/PRN473 blocks human CLEC-2, and PRN473 reduces venous thrombosis formation in mice
- PMID: 38968150
- PMCID: PMC11539360
- DOI: 10.1182/bloodadvances.2024012713
Selective Btk inhibition by PRN1008/PRN473 blocks human CLEC-2, and PRN473 reduces venous thrombosis formation in mice
Abstract
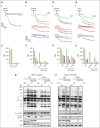
Platelet C-type lectin-like receptor 2 (CLEC-2) is a hem-immunoreceptor tyrosine-based activation motif-containing receptor that has a critical role in venous thrombosis but minimal involvement in hemostasis. CLEC-2 can be blocked by Btk inhibitors. Treatment with ibrutinib is associated with increased bleeding due to off-target inhibition of Src family kinases (SFKs). Patients with X-linked agammaglobulinemia (XLA) who lack Btk, however, do not bleed, suggesting selective Btk inhibition as a viable antithrombotic strategy. We assessed the effects of selective Btk inhibitors PRN1008 (rilzabrutinib) and PRN473 on platelet signaling and function mediated by CLEC-2 and glycoprotein-VI. We used healthy donors and XLA platelets to determine off-target inhibitor effects. Inferior vena cava (IVC) stenosis and Salmonella infection mouse models were used to assess antithrombotic effects of PRN473 in vivo. PRN1008 and PRN473 potently inhibited CLEC-2-mediated platelet activation to rhodocytin. No off-target inhibition of SFKs was seen. PRN1008 treatment of Btk-deficient platelets resulted in minor additional inhibition of aggregation and tyrosine phosphorylation, likely reflecting inhibition of Tec. No effect on G protein-coupled receptor-mediated platelet function was observed. PRN473 significantly reduced the number of thrombi in podoplanin-positive vessels after Salmonella infection and the presence of IVC thrombosis after vein stenosis. The potent inhibition of human platelet CLEC-2 and reduced thrombosis in in vivo models, together with the lack of off-target SFK inhibition and absence of bleeding reported in rilzabrutinib-treated patients with immune thrombocytopenia, suggest Btk inhibition as a promising antithrombotic strategy.
© 2024 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: P.L.R.N., M.R.T., and S.P.W. have received research grants from Novartis, Principia Biopharma, and Rigel Pharmaceuticals. P.L.R.N. has received research funding from AstraZeneca and honoraria from Bayer, Grifols, and Takeda. The remaining authors declare no competing financial interests.
Figures







References
-
- Villa E, Cammà C, Marietta M, et al. Enoxaparin prevents portal vein thrombosis and liver decompensation in patients with advanced cirrhosis. Gastroenterology. 2012;143(5):1253–1260.e4. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

