Homoharringtonine inhibits the NOTCH/MYC pathway and exhibits antitumor effects in T-cell acute lymphoblastic leukemia
- PMID: 38968151
- PMCID: PMC11451333
- DOI: 10.1182/blood.2023023400
Homoharringtonine inhibits the NOTCH/MYC pathway and exhibits antitumor effects in T-cell acute lymphoblastic leukemia
Abstract
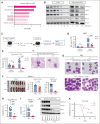
We report on the antileukemic activity of homoharringtonine (HHT) in T-cell acute lymphoblastic leukemia (T-ALL). We showed that HHT inhibited the NOTCH/MYC pathway and induced significantly longer survival in mouse and patient-derived T-ALL xenograft models, supporting HHT as a promising agent for T-ALL.
© 2024 American Society of Hematology. Published by Elsevier Inc. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures

References
-
- Nazha A, Kantarjian H, Cortes J, Quintas-Cardama A. Omacetaxine mepesuccinate (synribo) - newly launched in chronic myeloid leukemia. Expert Opin Pharmacother. 2013;14(14):1977–1986. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

