Infliximab in steroid-refractory immune-related hepatitis does not demonstrate hepatotoxicity and may shorten time on steroids
- PMID: 38969522
- PMCID: PMC11227818
- DOI: 10.1136/jitc-2023-008074
Infliximab in steroid-refractory immune-related hepatitis does not demonstrate hepatotoxicity and may shorten time on steroids
Abstract
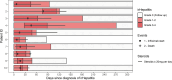
Background: Immune-related hepatitis (irHepatitis) is a relatively common immune-related adverse event (irAE) of checkpoint inhibitors. Often, it responds well to steroids; however, in refractory cases, further therapy is needed. Anti-tumor necrosis factor (TNF) antibodies are used for management of multiple irAEs, but there are little data in irHepatitis. Here, we report on safety and efficacy of infliximab in 10 cases of steroid-refractory irHepatitis.
Methods: We retrospectively reviewed patients treated with infliximab for steroid-refractory grade ≥3 irHepatitis at the Department of Dermatology, University Hospital Zurich. The positive response to infliximab was defined as no further increase in alanine aminotransferase (ALT)/aspartate aminotransferase (AST) above 50% than at the time of first infliximab infusion and control of irHepatitis without therapies other than steroids and infliximab.
Results: 10 patients with steroid-resistant irHepatitis grade ≥3 were treated with infliximab 5 mg/kg, of whom 7 (70%) responded positively. In two cases, the liver values increased over 50% before the irHepatitis could be controlled. In another case, therapies other than infliximab and steroids were given. At the median follow-up of 487 days, 90% of the patients demonstrated resolved irHepatitis without AST/ALT elevation following infliximab infusions.
Conclusions: Treatment of irHepatitis with infliximab did not result in hepatotoxicity and led to long-lasting positive response in 9 of 10 of the cases. Further research is needed to evaluate the role of anti-TNF antibodies in management of irHepatitis.
Keywords: Immune Checkpoint Inhibitors; Melanoma.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
