Nicotinamide N-methyltransferase inhibition mimics and boosts exercise-mediated improvements in muscle function in aged mice
- PMID: 38969654
- PMCID: PMC11226645
- DOI: 10.1038/s41598-024-66034-9
Nicotinamide N-methyltransferase inhibition mimics and boosts exercise-mediated improvements in muscle function in aged mice
Abstract
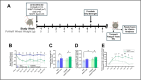
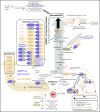
Human hallmarks of sarcopenia include muscle weakness and a blunted response to exercise. Nicotinamide N-methyltransferase inhibitors (NNMTis) increase strength and promote the regenerative capacity of aged muscle, thus offering a promising treatment for sarcopenia. Since human hallmarks of sarcopenia are recapitulated in aged (24-month-old) mice, we treated mice from 22 to 24 months of age with NNMTi, intensive exercise, or a combination of both, and compared skeletal muscle adaptations, including grip strength, longitudinal running capacity, plantarflexor peak torque, fatigue, and muscle mass, fiber type, cross-sectional area, and intramyocellular lipid (IMCL) content. Exhaustive proteome and metabolome analyses were completed to identify the molecular mechanisms underlying the measured changes in skeletal muscle pathophysiology. Remarkably, NNMTi-treated aged sedentary mice showed ~ 40% greater grip strength than sedentary controls, while aged exercised mice only showed a 20% increase relative to controls. Importantly, the grip strength improvements resulting from NNMTi treatment and exercise were additive, with NNMTi-treated exercised mice developing a 60% increase in grip strength relative to sedentary controls. NNMTi treatment also promoted quantifiable improvements in IMCL content and, in combination with exercise, significantly increased gastrocnemius fiber CSA. Detailed skeletal muscle proteome and metabolome analyses revealed unique molecular mechanisms associated with NNMTi treatment and distinct molecular mechanisms and cellular processes arising from a combination of NNMTi and exercise relative to those given a single intervention. These studies suggest that NNMTi-based drugs, either alone or combined with exercise, will be beneficial in treating sarcopenia and a wide range of age-related myopathies.
Keywords: Aging; Exercise; Muscle; NNMT; Nicotinamide N-methyltransferase; Proteomics.
© 2024. The Author(s).
Conflict of interest statement
SJ Watowich is the Founder of Ridgeline Therapeutics; AL Dimet-Wiley and H Neelakantan are employees of Ridgeline Therapeutics. All other authors declare no conflicts of interest.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

