3D engineered scaffold for large-scale Vigil immunotherapy production
- PMID: 38969656
- PMCID: PMC11226630
- DOI: 10.1038/s41598-024-65993-3
3D engineered scaffold for large-scale Vigil immunotherapy production
Abstract
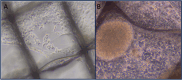
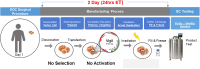
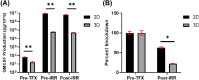
Previously, we reported successful cellular expansion of a murine colorectal carcinoma cell line (CT-26) using a three-dimensional (3D) engineered extracellular matrix (EECM) fibrillar scaffold structure. CCL-247 were grown over a limited time period of 8 days on 3D EECM or tissue culture polystyrene (TCPS). Cells were then assayed for growth, electroporation efficiency and Vigil manufacturing release criteria. Using EECM scaffolds, we report an expansion of CCL-247 (HCT116), a colorectal carcinoma cell line, from a starting concentration of 2.45 × 105 cells to 1.9 × 106 cells per scaffold. Following expansion, 3D EECM-derived cells were assessed based on clinical release criteria of the Vigil manufacturing process utilized for Phase IIb trial operation with the FDA. 3D EECM-derived cells passed all Vigil manufacturing release criteria including cytokine expression. Here, we demonstrate successful Vigil product manufacture achieving the specifications necessary for the clinical trial product release of Vigil treatment. Our results confirm that 3D EECM can be utilized for the expansion of human cancer cell CCL-247, justifying further clinical development involving human tissue sample manufacturing including core needle biopsy and minimal ascites samples.
Keywords: Autologous tumor cell therapy; Cell expansion; Immunotherapy; Ovarian cancer; Vigil.
© 2024. The Author(s).
Conflict of interest statement
This collaborative study has been supported by Gradalis. The University of Michigan has filed a patent application related to aspects of this work. JN is a board member and employee of Gradalis Inc. FK, EB, LS, HZ, CJ, and AW are employees of Gradalis, Inc. All other authors do not hold any competing interest.
Figures


References
-
- Maples P, Kumar P, Oxendine I, Jay C, Yu Y, Kuhn J, Nemunaitis J. TAG vaccine: Autologous tumor vaccine genetically modified to express GM-CSF and block production of TGFB2. BioProcess J. 2009;8(1):38–44. doi: 10.12665/J81.Maples.P2. - DOI
-
- Maples P, Kumar P, Yu Y, Wang Z, Jay C, Pappen B, et al. FANG vaccine: Autologous tumor cell vaccine genetically modified to express GM-CSF and block production of furin. BioProcess. J. 2010;8(4):4–14. doi: 10.12665/J84.Maples. - DOI
MeSH terms
LinkOut - more resources
Full Text Sources

