Homoharringtonine may help improve the outcomes of venetoclax and azacitidine in AML1-ETO positive acute myeloid leukemia
- PMID: 38969948
- PMCID: PMC11226518
- DOI: 10.1007/s00432-024-05861-9
Homoharringtonine may help improve the outcomes of venetoclax and azacitidine in AML1-ETO positive acute myeloid leukemia
Abstract
Purpose: T(8;21)(q22;q22.1)/AML1-ETO positive acute myeloid leukemia (AE-AML) is sensitive to conventional chemotherapy with a favorable prognosis. However, recent small case reports suggest the limited effectiveness of venetoclax (VEN) and hypomethylating agents (HMA) in treating AE-AML. The aim of this retrospective study was to evaluate the effectiveness of VEN plus AZA (VA) in AE-AML and explore whether adding homoharringtonine (HHT) to VA (VAH) could improve the response.
Methods: Patients who received VEN plus AZA and HHT (VAH) or VEN plus AZA (VA) regimens were included in this retrospective study. The endpoints of this study were to evaluate the rate of composite complete remission (CRc), measurable residual disease (MRD), event-free survival (EFS), overall survival (OS), and relapse between VAH and VA groups.
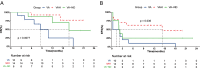
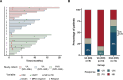
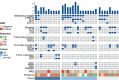
Results: A total of 32 AE-AML patients who underwent VA or VAH treatments (newly diagnosed with VA, ND-VA, n = 8; relapsed/refractory with VA, R/R-VA, n = 10; relapsed/refractory with VAH, R/R-VAH, n = 14) were included. The CR (complete remission) /CRi (CR with incomplete count recovery) rate of ND-VA, R/R-VA and R/R-VAH were 25%, 10%, and 64.3%, respectively. Measurable residual disease (MRD) negative was observed in 66.7% of R/R-VAH and none of VA-R/R patients. Co-occurring methylation mutations are associated with poor outcomes with VA but exhibit a more favorable response with VAH treatment. Additionally, patients with c-kit mutation presented inferior outcomes with both VEN-based regimens. All regimens were tolerated well by all patients.
Conclusion: Our data confirmed the poor response of VA in AE-AML, whether used as frontline or salvage therapy. Adding HHT to VA may improve outcomes and enhance the efficacy of VEN in this population.
Keywords: AML1/ETO positive; Acute myeloid leukemia; Azacitidine; Homoharringtonine; Venetoclax.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- DiNardo CD, Pratz KW, Letai A et al (2018) Safety and preliminary efficacy of venetoclax with decitabine or azacitidine in elderly patients with previously untreated acute myeloid leukaemia: a non-randomised, open-label, phase 1b study. Lancet Oncol Feb 19(2):216–228. 10.1016/s1470-2045(18)30010-x - DOI - PubMed
-
- National Comprehensive Cancer Network guidelines Acute myeloid leukemia Version 1.2023, https://www.nccn.org
-
- Konopleva M, Pollyea DA, Potluri J et al (2016) Efficacy and Biological correlates of response in a phase II study of Venetoclax Monotherapy in patients with Acute Myelogenous Leukemia. Cancer Discov Oct 6(10):1106–1117. 10.1158/2159-8290.Cd-16-0313 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- No. 82170215, 82293634, 81970161, 81870144, 82200241 and 82270178/National Natural Science Foundation of China
- No. 82170215, 82293634, 81970161, 81870144, 82200241 and 82270178/National Natural Science Foundation of China
- No. 2021YFC2500300-2021YFC2500302 and 2022YFA1105000/National Key Research and Development Program of China
- No. 2023A1515010111/Natural Science Foundation of Guangdong Province
LinkOut - more resources
Full Text Sources
Medical

