Interplay between WNT/PI3K-mTOR axis and the microbiota in APC-driven colorectal carcinogenesis: data from a pilot study and possible implications for CRC prevention
- PMID: 38970018
- PMCID: PMC11227240
- DOI: 10.1186/s12967-024-05305-5
Interplay between WNT/PI3K-mTOR axis and the microbiota in APC-driven colorectal carcinogenesis: data from a pilot study and possible implications for CRC prevention
Abstract
Background: Wnt/β-catenin signalling impairment accounts for 85% of colorectal cancers (CRCs), including sporadic and familial adenomatous polyposis (FAP) settings. An altered PI3K/mTOR pathway and gut microbiota also contribute to CRC carcinogenesis. We studied the interplay between the two pathways and the microbiota composition within each step of CRC carcinogenesis.
Methods: Proteins and target genes of both pathways were analysed by RT-qPCR and IHC in tissues from healthy faecal immunochemical test positive (FIT+, n = 17), FAP (n = 17) and CRC (n = 15) subjects. CRC-related mutations were analysed through NGS and Sanger. Oral, faecal and mucosal microbiota was profiled by 16 S rRNA-sequencing.
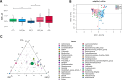
Results: We found simultaneous hyperactivation of Wnt/β-catenin and PI3K/mTOR pathways in FAP-lesions compared to CRCs. Wnt/β-catenin molecular markers positively correlated with Clostridium_sensu_stricto_1 and negatively with Bacteroides in FAP faecal microbiota. Alistipes, Lachnospiraceae, and Ruminococcaceae were enriched in FAP stools and adenomas, the latter also showing an overabundance of Lachnoclostridium, which positively correlated with cMYC. In impaired-mTOR-mutated CRC tissues, p-S6R correlated with Fusobacterium and Dialister, the latter also confirmed in the faecal-ecosystem.
Conclusions: Our study reveals an interplay between Wnt/β-catenin and PI3K/mTOR, whose derangement correlates with specific microbiota signatures in FAP and CRC patients, and identifies new potential biomarkers and targets to improve CRC prevention, early adenoma detection and treatment.
Keywords: Colorectal cancer; Familial adenomatous polyposis; Microbiota; PI3K/mTOR; Wnt/β-catenin.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no potential conflicts of interest.
Figures




Similar articles
-
Comparative Analysis of Commercial and Home-Made Media on RSPO1/S6R Axis in Organoids with Different Wnt Backgrounds: A Methodological Guide for the Selection of Intestinal Patient-Derived Organoids Culture Media.Int J Mol Sci. 2024 Oct 26;25(21):11526. doi: 10.3390/ijms252111526. Int J Mol Sci. 2024. PMID: 39519079 Free PMC article.
-
miR-155 Is Downregulated in Familial Adenomatous Polyposis and Modulates WNT Signaling by Targeting AXIN1 and TCF4.Mol Cancer Res. 2018 Dec;16(12):1965-1976. doi: 10.1158/1541-7786.MCR-18-0115. Epub 2018 Aug 2. Mol Cancer Res. 2018. PMID: 30072583
-
Analysis of K-ras, APC, and beta-catenin in aberrant crypt foci in sporadic adenoma, cancer, and familial adenomatous polyposis.Gastroenterology. 2001 Sep;121(3):599-611. doi: 10.1053/gast.2001.27203. Gastroenterology. 2001. PMID: 11522744
-
Molecular Pathways of Carcinogenesis in Familial Adenomatous Polyposis.Int J Mol Sci. 2023 Mar 16;24(6):5687. doi: 10.3390/ijms24065687. Int J Mol Sci. 2023. PMID: 36982759 Free PMC article. Review.
-
Familial adenomatous polyposis and changes in the gut microbiota: New insights into colorectal cancer carcinogenesis.World J Gastrointest Oncol. 2021 Jun 15;13(6):495-508. doi: 10.4251/wjgo.v13.i6.495. World J Gastrointest Oncol. 2021. PMID: 34163569 Free PMC article. Review.
Cited by
-
The role of gut microbiome in colorectal cancer development: a comprehensive analysis based on metabolomics and immunomodulatory mechanisms.Front Oncol. 2025 Jul 11;15:1629495. doi: 10.3389/fonc.2025.1629495. eCollection 2025. Front Oncol. 2025. PMID: 40718835 Free PMC article. Review.
-
Comparative Analysis of Commercial and Home-Made Media on RSPO1/S6R Axis in Organoids with Different Wnt Backgrounds: A Methodological Guide for the Selection of Intestinal Patient-Derived Organoids Culture Media.Int J Mol Sci. 2024 Oct 26;25(21):11526. doi: 10.3390/ijms252111526. Int J Mol Sci. 2024. PMID: 39519079 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

