The crosstalk between copper-induced oxidative stress and cuproptosis: a novel potential anticancer paradigm
- PMID: 38970072
- PMCID: PMC11225285
- DOI: 10.1186/s12964-024-01726-3
The crosstalk between copper-induced oxidative stress and cuproptosis: a novel potential anticancer paradigm
Abstract
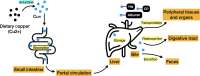
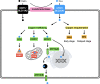
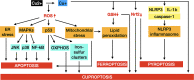
Copper is a crucial trace element that plays a role in various pathophysiological processes in the human body. Copper also acts as a transition metal involved in redox reactions, contributing to the generation of reactive oxygen species (ROS). Under prolonged and increased ROS levels, oxidative stress occurs, which has been implicated in different types of regulated cell death. The recent discovery of cuproptosis, a copper-dependent regulated cell death pathway that is distinct from other known regulated cell death forms, has raised interest to researchers in the field of cancer therapy. Herein, the present work aims to outline the current understanding of cuproptosis, with an emphasis on its anticancer activities through the interplay with copper-induced oxidative stress, thereby providing new ideas for therapeutic approaches targeting modes of cell death in the future.
Keywords: Copper; Cuproptosis; Oxidative stress; Reactive oxygen species.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Tsvetkov P, Coy S, Petrova B, Dreishpoon M, Verma A, Abdusamad M, Rossen J, Joesch-Cohen L, Humeidi R, Spangler RD, Eaton JK, Frenkel E, Kocak M, Corsello SM, Lutsenko S, Kanarek N, Santagata S. Golub T.R. Copper induces cell death by targeting lipoylated TCA cycle proteins. Sci (New York) 2022;375(6586):1254–61. doi: 10.1126/science.abf0529. - DOI - PMC - PubMed
-
- World Health Organization. Global cancer burden growing, amidst mounting need for services. 2024. https://www.who.int/news/item/01-02-2024-global-cancer-burden-growing--a... (Accessed on 04 April 2024). - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

