Neuroimmune modulating and energy supporting nanozyme-mimic scaffold synergistically promotes axon regeneration after spinal cord injury
- PMID: 38970101
- PMCID: PMC11225227
- DOI: 10.1186/s12951-024-02594-2
Neuroimmune modulating and energy supporting nanozyme-mimic scaffold synergistically promotes axon regeneration after spinal cord injury
Abstract
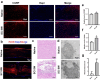
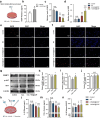
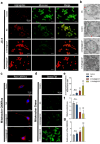
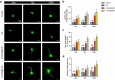
Spinal cord injury (SCI) represents a profound central nervous system affliction, resulting in irreversibly compromised daily activities and disabilities. SCI involves excessive inflammatory responses, which are characterized by the existence of high levels of proinflammatory M1 macrophages, and neuronal mitochondrial energy deficit, exacerbating secondary damage and impeding axon regeneration. This study delves into the mechanistic intricacies of SCI, offering insights from the perspectives of neuroimmune regulation and mitochondrial function, leading to a pro-fibrotic macrophage phenotype and energy-supplying deficit. To address these challenges, we developed a smart scaffold incorporating enzyme mimicry nanoparticle-ceriumoxide (COPs) into nanofibers (NS@COP), which aims to pioneer a targeted neuroimmune repair strategy, rescuing CGRP receptor on macrophage and concurrently remodeling mitochondrial function. Our findings indicate that the integrated COPs restore the responsiveness of pro-inflammatory macrophages to calcitonin gene-related peptide (CGRP) signal by up-regulating receptor activity modifying protein 1 (RAMP1), a vital component of the CGRP receptor. This promotes macrophage fate commitment to an anti-inflammatory pro-resolution M2 phenotype, then alleviating glial scar formation. In addition, NS@COP implantation also protected neuronal mitochondrial function. Collectively, our results suggest that the strategy of integrating nanozyme COP nanoparticles into a nanofiber scaffold provides a promising therapeutic candidate for spinal cord trauma via rational regulation of neuroimmune communication and mitochondrial function.
Keywords: Axonal regeneration; Calcitonin gene-related peptide; Cerium oxide nanoparticles; Mitochondria; Polarization of macrophages; Reactive oxygen species.
© 2024. The Author(s).
Conflict of interest statement
The authors have declared that no conflicts of interest exist.
Figures





References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

