Detection of colorectal-cancer-associated bacterial taxa in fecal samples using next-generation sequencing and 19 newly established qPCR assays
- PMID: 38970464
- PMCID: PMC11793011
- DOI: 10.1002/1878-0261.13700
Detection of colorectal-cancer-associated bacterial taxa in fecal samples using next-generation sequencing and 19 newly established qPCR assays
Abstract
We have previously identified increased levels of distinct bacterial taxa within mucosal biopsies from colorectal cancer (CRC) patients. Following prior research, the aim of this study was to investigate the detection of the same CRC-associated bacteria in fecal samples and to evaluate the suitability of fecal samples as a non-invasive material for the detection of CRC-associated bacteria. Next-generation sequencing (NGS) of the 16S ribosomal RNA (rRNA) V4 region was performed to evaluate the detection of the CRC-associated bacteria in the fecal microbiota of cancer patients, patients with adenomatous polyp and healthy controls. Furthermore, 19 novel species-specific quantitative PCR (qPCR) assays were established to detect the CRC-associated bacteria. Approximately, 75% of the bacterial taxa identified in biopsies were reflected in fecal samples. NGS failed to detect low-abundance CRC-associated taxa in fecal samples, whereas qPCR exhibited high sensitivity and specificity in identifying all targeted taxa. Comparison of fecal microbial composition between the different patient groups showed enrichment of Fusobacterium nucleatum, Parvimonas micra, and Gemella morbillorum in cancer patients. Our findings suggest that low-abundance mucosa-associated bacteria can be detected in fecal samples using sensitive qPCR assays.
Keywords: 16S rRNA amplicon sequencing; colorectal cancer; contaminant species; fecal microbiota; mucosal microbiota; qPCR.
© 2024 The Author(s). Molecular Oncology published by John Wiley & Sons Ltd on behalf of Federation of European Biochemical Societies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


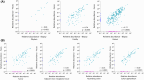

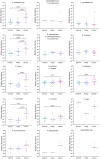
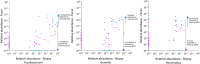
References
-
- Morgan E, Arnold M, Gini A, Lorenzoni V, Cabasag CJ, Laversanne M, et al. Global burden of colorectal cancer in 2020 and 2040: incidence and mortality estimates from GLOBOCAN. Gut. 2023;72(2):338–344. - PubMed
-
- Kronborg O, Fenger C, Olsen J, Jørgensen OD, Søndergaard O. Randomised study of screening for colorectal cancer with faecal‐occult‐blood test. Lancet. 1996;348(9040):1467–1471. - PubMed
-
- Bretthauer M, Løberg M, Wieszczy P, Kalager M, Emilsson L, Garborg K, et al. Effect of colonoscopy screening on risks of colorectal cancer and related death. N Engl J Med. 2022;387(17):1547–1556. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

