Natural Products Have Increased Rates of Clinical Trial Success throughout the Drug Development Process
- PMID: 38970498
- PMCID: PMC11287737
- DOI: 10.1021/acs.jnatprod.4c00581
Natural Products Have Increased Rates of Clinical Trial Success throughout the Drug Development Process
Abstract
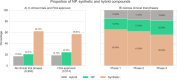
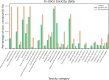
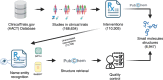
Natural products (NPs) or their derivatives represent a large proportion of drugs that successfully progress through clinical trials to approval. This study explores the presence of NPs in both early- and late-stage drug discovery to determine their success rate, and the factors or features of natural products that contribute to such success. As a proxy for early drug development stages, we analyzed patent applications over several decades, finding a consistent proportion of NP, NP-derived, and synthetic-compound-based patent documents, with the latter group outnumbering NP and NP-derived ones (approximately 77% vs 23%). We next assessed clinical trial data, where we observed a steady increase in NP and NP-derived compounds from clinical trial phases I to III (from approximately 35% in phase I to 45% in phase III), with an inverse trend observed in synthetics (from approximately 65% in phase I to 55% in phase III). Finally, in vitro and in silico toxicity studies revealed that NPs and their derivatives were less toxic alternatives to their synthetic counterparts. These discoveries offer valuable insights for successful NP-based drug development, highlighting the potential benefits of prioritizing NPs and their derivatives as starting points.
Conflict of interest statement
The authors declare the following competing financial interest(s): All authors were employees of Enveda Biosciences Inc. during the course of this work and have a real or potential ownership interest in Enveda Biosciences Inc.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

