Endogenous LXR signaling controls pulmonary surfactant homeostasis and prevents lung inflammation
- PMID: 38970705
- PMCID: PMC11335212
- DOI: 10.1007/s00018-024-05310-3
Endogenous LXR signaling controls pulmonary surfactant homeostasis and prevents lung inflammation
Abstract
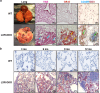
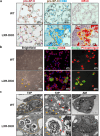
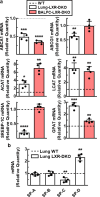
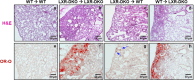
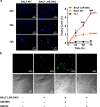
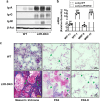
Lung type 2 pneumocytes (T2Ps) and alveolar macrophages (AMs) play crucial roles in the synthesis, recycling and catabolism of surfactant material, a lipid/protein fluid essential for respiratory function. The liver X receptors (LXR), LXRα and LXRβ, are transcription factors important for lipid metabolism and inflammation. While LXR activation exerts anti-inflammatory actions in lung injury caused by lipopolysaccharide (LPS) and other inflammatory stimuli, the full extent of the endogenous LXR transcriptional activity in pulmonary homeostasis is incompletely understood. Here, using mice lacking LXRα and LXRβ as experimental models, we describe how the loss of LXRs causes pulmonary lipidosis, pulmonary congestion, fibrosis and chronic inflammation due to defective de novo synthesis and recycling of surfactant material by T2Ps and defective phagocytosis and degradation of excess surfactant by AMs. LXR-deficient T2Ps display aberrant lamellar bodies and decreased expression of genes encoding for surfactant proteins and enzymes involved in cholesterol, fatty acids, and phospholipid metabolism. Moreover, LXR-deficient lungs accumulate foamy AMs with aberrant expression of cholesterol and phospholipid metabolism genes. Using a house dust mite aeroallergen-induced mouse model of asthma, we show that LXR-deficient mice exhibit a more pronounced airway reactivity to a methacholine challenge and greater pulmonary infiltration, indicating an altered physiology of LXR-deficient lungs. Moreover, pretreatment with LXR agonists ameliorated the airway reactivity in WT mice sensitized to house dust mite extracts, confirming that LXR plays an important role in lung physiology and suggesting that agonist pharmacology could be used to treat inflammatory lung diseases.
Keywords: Alveolar macrophage; Inflammation; LXR; Lipidosis; Surfactant; Type 2 pneumocyte.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

