Mesonephric-type adenocarcinomas of the ovary: prevalence, diagnostic reproducibility, outcome, and value of PAX2
- PMID: 38970797
- PMCID: PMC11227277
- DOI: 10.1002/2056-4538.12389
Mesonephric-type adenocarcinomas of the ovary: prevalence, diagnostic reproducibility, outcome, and value of PAX2
Abstract
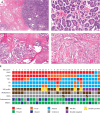
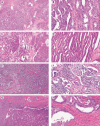
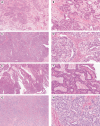
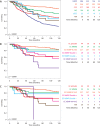
Mesonephric-type (or -like) adenocarcinomas (MAs) of the ovary are an uncommon and aggressive histotype. They appear to arise through transdifferentiation from Müllerian lesions creating diagnostic challenges. Thus, we aimed to develop a histologic and immunohistochemical (IHC) approach to optimize the identification of MA over its histologic mimics, such as ovarian endometrioid carcinoma (EC). First, we screened 1,537 ovarian epithelial neoplasms with a four-marker IHC panel of GATA3, TTF1, ER, and PR followed by a morphological review of EC to identify MA in retrospective cohorts. Interobserver reproducibility for the distinction of MA versus EC was assessed in 66 cases initially without and subsequently with IHC information (four-marker panel). Expression of PAX2, CD10, and calretinin was evaluated separately, and survival analyses were performed. We identified 23 MAs from which 22 were among 385 cases initially reported as EC (5.7%) and 1 as clear cell carcinoma. The interobserver reproducibility increased from fair to substantial (κ = 0.376-0.727) with the integration of the four-marker IHC panel. PAX2 was the single most sensitive and specific marker to distinguish MA from EC and could be used as a first-line marker together with ER/PR and GATA3/TTF1. Patients with MA had significantly increased risk of earlier death from disease (hazard ratio = 3.08; 95% CI, 1.62-5.85; p < 0.0001) compared with patients with EC, when adjusted for age, stage, and p53 status. A diagnosis of MA has prognostic implications for stage I disease, and due to the subtlety of morphological features in some tumors, a low threshold for ancillary testing is recommended.
Keywords: GATA3; PAX2; TTF1; mesonephric; mesonephric‐like.
© 2024 The Author(s). The Journal of Pathology: Clinical Research published by The Pathological Society of Great Britain and Ireland and John Wiley & Sons Ltd.
Figures

References
-
- McCluggage WG. Mesonephric‐like adenocarcinoma of the female genital tract: from morphologic observations to a well‐characterized carcinoma with aggressive clinical behavior. Adv Anat Pathol 2022; 29: 208–216. - PubMed
-
- McFarland M, Quick CM, McCluggage WG. Hormone receptor‐negative, thyroid transcription factor 1‐positive uterine and ovarian adenocarcinomas: report of a series of mesonephric‐like adenocarcinomas. Histopathology 2016; 68: 1013–1020. - PubMed
-
- WHO Classification of Tumours Editorial Board . Female Genital Tumours (5th edn). International Agency for Research on Cancer: Lyon, 2020.
-
- McCluggage WG, Vosmikova H, Laco J. Ovarian combined low‐grade serous and mesonephric‐like adenocarcinoma: further evidence for a Mullerian origin of mesonephric‐like adenocarcinoma. Int J Gynecol Pathol 2020; 39: 84–92. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

