Cellular and molecular mechanisms of hepatic ischemia-reperfusion injury: The role of oxidative stress and therapeutic approaches
- PMID: 38970988
- PMCID: PMC11279328
- DOI: 10.1016/j.redox.2024.103258
Cellular and molecular mechanisms of hepatic ischemia-reperfusion injury: The role of oxidative stress and therapeutic approaches
Abstract
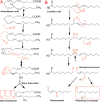
Ischemia-reperfusion (IR) or reoxygenation injury is the paradoxical exacerbation of cellular impairment following restoration of blood flow after a period of ischemia during surgical procedures or other conditions. Acute interruption of blood supply to the liver and subsequent reperfusion can result in hepatocyte injury, apoptosis, and necrosis. Since the liver requires a continuous supply of oxygen for many biochemical reactions, any obstruction of blood flow can rapidly lead to hepatic hypoxia, which could quickly progress to absolute anoxia. Reoxygenation results in the increased generation of reactive oxygen species and oxidative stress, which lead to the enhanced production of proinflammatory cytokines, chemokines, and other signaling molecules. Consequent acute inflammatory cascades lead to significant impairment of hepatocytes and nonparenchymal cells. Furthermore, the expression of several vascular growth factors results in the heterogeneous closure of numerous hepatic sinusoids, which leads to reduced oxygen supply in certain areas of the liver even after reperfusion. Therefore, it is vital to identify appropriate therapeutic modalities to mitigate hepatic IR injury and subsequent tissue damage. This review covers all the major aspects of cellular and molecular mechanisms underlying the pathogenesis of hepatic ischemia-reperfusion injury, with special emphasis on oxidative stress, associated inflammation and complications, and prospective therapeutic approaches.
Keywords: 4-Hydroxy-2-nonenal; Free radicals; Ischemia-reperfusion injury; Malondialdehyde; Oxidative stress; γ-glutamyl transpeptidase.
Copyright © 2024 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures





References
-
- Beltran-Camacho L., Jimenez-Palomares M., Rojas-Torres M., Sanchez-Gomar I., Rosal-Vela A.…Durán-Ruiz M.C. Identification of the initial molecular changes in response to circulating angiogenic cells-mediated therapy in critical limb ischemia. Stem Cell Res. Ther. 2020;11(1):106. doi: 10.1186/s13287-020-01591-0. PMID: 32143690. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

