A toolkit for a modern gynecologic oncology tissue bank
- PMID: 38971004
- PMCID: PMC12052217
- DOI: 10.1016/j.ygyno.2024.07.001
A toolkit for a modern gynecologic oncology tissue bank
Abstract
Objectives: Tissue banking procedures have evolved to keep pace with precision medicine, technology, emerging understanding of racial disparities, and regulatory requirements. However, there is little published guidance regarding strategies to create and maintain a successful biorepository. Our objective is to describe the infrastructure and protocols used by our Gynecologic Oncology Tissue Bank.
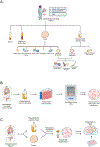
Methods: Our Tissue Bank was founded in 1992. In August 2022, internal funding was used to modernize the Tissue Bank. We hired three full-time employees, implemented universal screening of patients treated by gynecologic oncology faculty, updated consenting protocols, and standardized communication with providers. Tumor tissue, blood derivatives, ascites, and pleural fluid were collected from eligible, consenting patients and processed. Patient-derived cell lines and organoids were generated. For quality control purposes, one formalin-fixed, paraffin-embedded (FFPE) sample per tissue site was analyzed by a board-certified pathologist. All samples were labeled and tracked in an OpenSpecimen collection protocol and clinically annotated in a secure database.
Results: From August 2022 to October 2023, 227 patients (83% white, 15% Black, 1% Asian) were enrolled and 4249 specimens were collected. Adherent cell lines were generated from 15 patients with ovarian cancer and cell suspensions for organoid generation were collected from 46 patients with ovarian cancer. A recharge center was established to self-sustain the Tissue Bank. Samples have been shared with academic and commercial collaborators.
Conclusions: Our Tissue Bank has enrolled a large number of diverse patients, collected numerous specimen types, and collaborated widely. The procedures described here provide guidance for other institutions establishing similar resources.
Keywords: Biorepository; Endometrial cancer; Gynecologic oncology; Ovarian cancer; Tissue bank.
Copyright © 2024 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors have no conflicts of interest.
Figures

References
-
- Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73(1):17–48. - PubMed
-
- Compadre AJ, van Biljon LN, Valentine MC, Llop-Guevara A, Graham E, Fashemi B, et al. RAD51 Foci as a Biomarker Predictive of Platinum Chemotherapy Response in Ovarian Cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2023;29(13):2466–79. - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

