The art of designed coiled-coils for the regulation of mammalian cells
- PMID: 38971158
- PMCID: PMC11335187
- DOI: 10.1016/j.chembiol.2024.06.001
The art of designed coiled-coils for the regulation of mammalian cells
Abstract
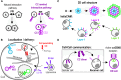
Synthetic biology aims to engineer complex biological systems using modular elements, with coiled-coil (CC) dimer-forming modules are emerging as highly useful building blocks in the regulation of protein assemblies and biological processes. Those small modules facilitate highly specific and orthogonal protein-protein interactions, offering versatility for the regulation of diverse biological functions. Additionally, their design rules enable precise control and tunability over these interactions, which are crucial for specific applications. Recent advancements showcase their potential for use in innovative therapeutic interventions and biomedical applications. In this review, we discuss the potential of CCs, exploring their diverse applications in mammalian cells, such as synthetic biological circuit design, transcriptional and allosteric regulation, cellular assemblies, chimeric antigen receptor (CAR) T cell regulation, and genome editing and their role in advancing the understanding and regulation of cellular processes.
Copyright © 2024 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures





References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

