Aerobic exercise improves cognitive flexibility and modulates regional volume changes in a rat model of autism
- PMID: 38971431
- PMCID: PMC12035974
- DOI: 10.1016/j.bbr.2024.115136
Aerobic exercise improves cognitive flexibility and modulates regional volume changes in a rat model of autism
Abstract
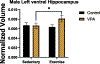
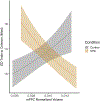
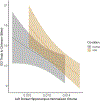
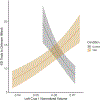
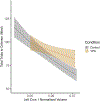
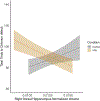
Gestational exposure to valproic acid (VPA) is a risk factor for autism spectrum disorder (ASD). Rodents exposed to VPA in utero display common features of ASD, including volumetric dysregulation in higher-order cognitive regions like the medial prefrontal cortex (mPFC), the anterior cingulate cortex (ACC), and the hippocampus. Exercise has been shown in elderly populations to boost cognition and to buffer against brain volume losses with age. This study employed an adolescent treadmill exercise intervention to facilitate cognitive flexibility and regional brain volume regulation in rats exposed to VPA during gestation. It was found that exercise improved performance on extra-dimensional shifts of attention on a set-shifting task, which is indicative of improved cognitive flexibility. Exercise decreased frontal cortex volume in females, whereas in males exercise increased the ventral hippocampus. These findings suggest that aerobic exercise may be an effective intervention to counteract the altered development of prefrontal and hippocampal regions often observed in ASD.
Keywords: ASD; Adolescence; Anterior cingulate; Brain volume; Cerebellum; Hippocampus; VPA.
Copyright © 2024. Published by Elsevier B.V.
Figures
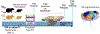
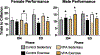
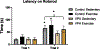
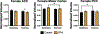
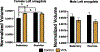
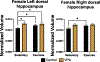
Similar articles
-
Region-Specific Brain Volume Changes Emerge in Adolescence in the Valproic Acid Model of Autism and Parallel Human Findings.Dev Neurosci. 2025;47(1):68-80. doi: 10.1159/000538932. Epub 2024 Apr 26. Dev Neurosci. 2025. PMID: 38679020 Free PMC article.
-
Effects of Cannabidiol Isolated or in Association With Risperidone in an Animal Model of Autism.Dev Neurobiol. 2025 Jan;85(1):e22955. doi: 10.1002/dneu.22955. Dev Neurobiol. 2025. PMID: 39604124
-
Lipopolysaccharide induces neuroinflammation in a valproic acid male model of autism.Brain Res Bull. 2025 Jan;220:111154. doi: 10.1016/j.brainresbull.2024.111154. Epub 2024 Nov 30. Brain Res Bull. 2025. PMID: 39622390
-
Differently different?: A commentary on the emerging social cognitive neuroscience of female autism.Biol Sex Differ. 2024 Jun 13;15(1):49. doi: 10.1186/s13293-024-00621-3. Biol Sex Differ. 2024. PMID: 38872228 Free PMC article. Review.
-
Treatment for epilepsy in pregnancy: neurodevelopmental outcomes in the child.Cochrane Database Syst Rev. 2014 Oct 30;2014(10):CD010236. doi: 10.1002/14651858.CD010236.pub2. Cochrane Database Syst Rev. 2014. PMID: 25354543 Free PMC article.
References
-
- Van Rooij D, Anagnostou E, Arango C, Auzias G, Behrmann M, Busatto GF, Calderoni S, Daly E, Deruelle C, Di Martino A, Dinstein I, Duran FLS, Durston S, Ecker C, Fair D, Fedor J, Fitzgerald J, Freitag CM, Gallagher L, Gori I, Haar S, Hoekstra L, Jahanshad N, Jalbrzikowski M, Janssen J, Lerch J, Luna B, Martinho MM, McGrath J, Muratori F, Murphy CM, Murphy DGM, O’Hearn K, Oranje B, Parellada M, Retico A, Rosa P, Rubia K, Shook D, Taylor M, Thompson PM, Tosetti M, Wallace GL, Zhou F, Buitelaar JK, Cortical and subcortical brain morphometry differences between patients with autism spectrum disorder and healthy individuals across the lifespan: results from the ENIGMA ASD working group, Am. J. Psychiatry 175 (2018) 359–369, 10.1176/appi.ajp.2017.17010100. - DOI - PMC - PubMed
-
- Schumann CM, Hamstra J, Goodlin-Jones BL, Lotspeich LJ, Kwon H, Buonocore MH, Lammers CR, Reiss AL, Amaral DG, The amygdala is enlarged in children but not adolescents with autism; the hippocampus is enlarged at all ages, J. Neurosci. Off. J. Soc. Neurosci. 24 (2004) 6392–6401, 10.1523/JNEUROSCI.1297-04.2004. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

