Essential role of p21Waf1/Cip1 in the modulation of post-traumatic hippocampal Neural Stem Cells response
- PMID: 38971774
- PMCID: PMC11227726
- DOI: 10.1186/s13287-024-03787-0
Essential role of p21Waf1/Cip1 in the modulation of post-traumatic hippocampal Neural Stem Cells response
Abstract
Background: Traumatic Brain Injury (TBI) represents one of the main causes of brain damage in young people and the elderly population with a very high rate of psycho-physical disability and death. TBI is characterized by extensive cell death, tissue damage and neuro-inflammation with a symptomatology that varies depending on the severity of the trauma from memory loss to a state of irreversible coma and death. Recently, preclinical studies on mouse models have demonstrated that the post-traumatic adult Neural Stem/Progenitor cells response could represent an excellent model to shed light on the neuro-reparative role of adult neurogenesis following damage. The cyclin-dependent kinase inhibitor p21Waf1/Cip1 plays a pivotal role in modulating the quiescence/activation balance of adult Neural Stem Cells (aNSCs) and in restraining the proliferation progression of progenitor cells. Based on these considerations, the aim of this work is to evaluate how the conditional ablation of p21Waf1/Cip1 in the aNSCS can alter the adult hippocampal neurogenesis in physiological and post-traumatic conditions.
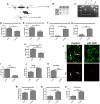
Methods: We designed a novel conditional p21Waf1/Cip1 knock-out mouse model, in which the deletion of p21Waf1/Cip1 (referred as p21) is temporally controlled and occurs in Nestin-positive aNSCs, following administration of Tamoxifen. This mouse model (referred as p21 cKO mice) was subjected to Controlled Cortical Impact to analyze how the deletion of p21 could influence the post-traumatic neurogenic response within the hippocampal niche.
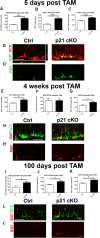
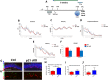
Results: The data demonstrates that the conditional deletion of p21 in the aNSCs induces a strong increase in activation of aNSCs as well as proliferation and differentiation of neural progenitors in the adult dentate gyrus of the hippocampus, resulting in an enhancement of neurogenesis and the hippocampal-dependent working memory. However, following traumatic brain injury, the increased neurogenic response of aNSCs in p21 cKO mice leads to a fast depletion of the aNSCs pool, followed by declined neurogenesis and impaired hippocampal functionality.
Conclusions: These data demonstrate for the first time a fundamental role of p21 in modulating the post-traumatic hippocampal neurogenic response, by the regulation of the proliferative and differentiative steps of aNSCs/progenitor populations after brain damage.
Keywords: Adult Neural Stem Cells; Adult hippocampal neurogenesis; Neural regeneration; Traumatic brain injury; Working memory; p21.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

