The human VGLUT3-pT8I mutation elicits uneven striatal DA signaling, food or drug maladaptive consumption in male mice
- PMID: 38971801
- PMCID: PMC11227582
- DOI: 10.1038/s41467-024-49371-1
The human VGLUT3-pT8I mutation elicits uneven striatal DA signaling, food or drug maladaptive consumption in male mice
Abstract
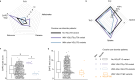
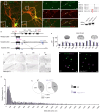
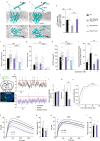
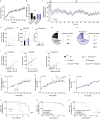
Cholinergic striatal interneurons (ChIs) express the vesicular glutamate transporter 3 (VGLUT3) which allows them to regulate the striatal network with glutamate and acetylcholine (ACh). In addition, VGLUT3-dependent glutamate increases ACh vesicular stores through vesicular synergy. A missense polymorphism, VGLUT3-p.T8I, was identified in patients with substance use disorders (SUDs) and eating disorders (EDs). A mouse line was generated to understand the neurochemical and behavioral impact of the p.T8I variant. In VGLUT3T8I/T8I male mice, glutamate signaling was unchanged but vesicular synergy and ACh release were blunted. Mutant male mice exhibited a reduced DA release in the dorsomedial striatum but not in the dorsolateral striatum, facilitating habit formation and exacerbating maladaptive use of drug or food. Increasing ACh tone with donepezil reversed the self-starvation phenotype observed in VGLUT3T8I/T8I male mice. Our study suggests that unbalanced dopaminergic transmission in the dorsal striatum could be a common mechanism between SUDs and EDs.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
MeSH terms
Substances
Grants and funding
- RGPIN/386431-2012/Gouvernement du Canada | Natural Sciences and Engineering Research Council of Canada (Conseil de Recherches en Sciences Naturelles et en Génie du Canada)
- RGPIN/04682-2017/Gouvernement du Canada | Natural Sciences and Engineering Research Council of Canada (Conseil de Recherches en Sciences Naturelles et en Génie du Canada)
LinkOut - more resources
Full Text Sources

