Modulating social learning-induced evaluation updating during human sleep
- PMID: 38971834
- PMCID: PMC11227583
- DOI: 10.1038/s41539-024-00255-5
Modulating social learning-induced evaluation updating during human sleep
Abstract
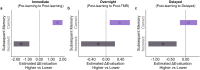
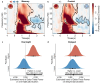
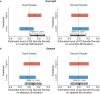
People often change their evaluations upon learning about their peers' evaluations, i.e., social learning. Given sleep's vital role in consolidating daytime experiences, sleep may facilitate social learning, thereby further changing people's evaluations. Combining a social learning task and the sleep-based targeted memory reactivation technique, we asked whether social learning-induced evaluation updating can be modulated during sleep. After participants had indicated their initial evaluation of snacks, they learned about their peers' evaluations while hearing the snacks' spoken names. During the post-learning non-rapid-eye-movement sleep, we re-played half of the snack names (i.e., cued snack) to reactivate the associated peers' evaluations. Upon waking up, we found that the social learning-induced evaluation updating further enlarged for both cued and uncued snacks. Examining sleep electroencephalogram (EEG) activity revealed that cue-elicited delta-theta EEG power and the overnight N2 sleep spindle density predicted post-sleep evaluation updating for cued but not for uncued snacks. These findings underscore the role of sleep-mediated memory reactivation and the associated neural activity in supporting social learning-induced evaluation updating.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Updating memories of unwanted emotions during human sleep.Curr Biol. 2023 Jan 23;33(2):309-320.e5. doi: 10.1016/j.cub.2022.12.004. Epub 2022 Dec 29. Curr Biol. 2023. PMID: 36584677 Free PMC article.
-
Targeted Memory Reactivation During Sleep Improves Next-Day Problem Solving.Psychol Sci. 2019 Nov;30(11):1616-1624. doi: 10.1177/0956797619873344. Epub 2019 Oct 11. Psychol Sci. 2019. PMID: 31603738 Free PMC article.
-
Targeted Memory Reactivation during Sleep Depends on Prior Learning.Sleep. 2015 May 1;38(5):755-63. doi: 10.5665/sleep.4670. Sleep. 2015. PMID: 25515103 Free PMC article. Clinical Trial.
-
Sleep, learning, and memory in human research using noninvasive neuroimaging techniques.Neurosci Res. 2023 Apr;189:66-74. doi: 10.1016/j.neures.2022.12.013. Epub 2022 Dec 23. Neurosci Res. 2023. PMID: 36572251 Review.
-
Role of normal sleep and sleep apnea in human memory processing.Nat Sci Sleep. 2018 Sep 4;10:255-269. doi: 10.2147/NSS.S125299. eCollection 2018. Nat Sci Sleep. 2018. PMID: 30214331 Free PMC article. Review.
Cited by
-
Personalized targeted memory reactivation enhances consolidation of challenging memories via slow wave and spindle dynamics.NPJ Sci Learn. 2025 Jul 22;10(1):47. doi: 10.1038/s41539-025-00340-3. NPJ Sci Learn. 2025. PMID: 40691439 Free PMC article.
References
-
- Hütter M. An integrative review of dual- and single-process accounts of evaluative conditioning. Nat. Rev. Psychol. 2022;1:640–653. doi: 10.1038/s44159-022-00102-7. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

