Production and stability of cultured red blood cells depends on the concentration of cholesterol in culture medium
- PMID: 38971841
- PMCID: PMC11227516
- DOI: 10.1038/s41598-024-66440-z
Production and stability of cultured red blood cells depends on the concentration of cholesterol in culture medium
Abstract
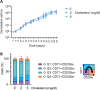
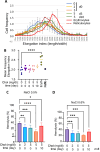
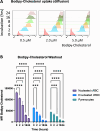
The production of cultured red blood cells (cRBC) for transfusion purposes requires large scale cultures and downstream processes to purify enucleated cRBC. The membrane composition, and cholesterol content in particular, are important during proliferation of (pro)erythroblasts and for cRBC quality. Therefore, we tested the requirement for cholesterol in the culture medium during expansion and differentiation of erythroid cultures with respect to proliferation, enucleation and purification by filtration. The low cholesterol level (22 µg/dl) in serum free medium was sufficient to expand (pro)erythroblast cultures. Addition of 2.0 or 5.0 mg/dL of free cholesterol at the start of differentiation induction inhibited enucleation compared to the default condition containing 3.3 mg/dl total cholesterol derived from the addition of Omniplasma to serum free medium. Addition of 5.0 mg/dl cholesterol at day 5 of differentiation did not affect the enucleation process but significantly increased recovery of enucleated cRBC following filtration over leukodepletion filters. The addition of cholesterol at day 5 increased the osmotic resistance of cRBC. In conclusion, cholesterol supplementation after the onset of enucleation improved the robustness of cRBC and increased the yield of enucleated cRBC in the purification process.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Knowles S. Blood transfusion: Challenges and limitations. Transfus. Altern. Transfus. Med. 2007;9:2–9. doi: 10.1111/j.1778-428X.2007.00062.x. - DOI
-
- Landsteiner K. Zur Kenntnis der antifermentativen, lytischen und agglutinierenden Wirkungen des Blutserums und der Lymphe. Zentralblatt fur Bakteriol. Parasitenkd. und Infekt. 1900;27:357–362.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

