Differences Between RSV A and RSV B Subgroups and Implications for Pharmaceutical Preventive Measures
- PMID: 38971918
- PMCID: PMC11266343
- DOI: 10.1007/s40121-024-01012-2
Differences Between RSV A and RSV B Subgroups and Implications for Pharmaceutical Preventive Measures
Abstract
Introduction: Understanding the differences between respiratory syncytial virus (RSV) subgroups A and B provides insights for the development of prevention strategies and public health interventions. We aimed to describe the structural differences of RSV subgroups, their epidemiology, and genomic diversity. The associated immune response and differences in clinical severity were also investigated.
Methods: A literature review from PubMed and Google Scholar (1985-2023) was performed and extended using snowballing from references in captured publications.
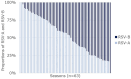
Results: RSV has two major antigenic subgroups, A and B, defined by the G glycoprotein. The RSV F fusion glycoprotein in the prefusion conformation is a major target of virus neutralizing antibodies and differs in surface exposed regions between RSV A and RSV B. The subgroups co-circulate annually, but there is considerable debate as to whether clinical severity is impacted by the subgroup of the infecting RSV strain. Large variations between the studies reporting RSV subgroup impact on clinical severity were observed. A tendency for higher disease severity may be attributed to RSV A but no consensus could be reached as to whether infection by one of the subgroup caused more severe outcomes. RSV genotype diversity decreased over the last two decades, and ON and BA have become the sole lineages detected for RSV A and RSV B, since 2014. No studies with data obtained after 2014 reported a difference in disease severity between the two subgroups. RSV F is relatively well conserved and highly similar between RSV A and B, but changes in the amino acid sequence have been observed. Some of these changes led to differences in F antigenic sites compared to reference F sequences (e.g., RSV/A Long strain), which are more pronounced in antigenic sites of the prefusion conformation of RSV B. Initial results from the second season after vaccination suggest specific RSV B efficacy wanes more rapidly than RSV A for RSV PreF-based monovalent vaccines.
Conclusions: RSV A and RSV B both contribute substantially to the global RSV burden. Both RSV subgroups cause severe disease and none of the available evidence to date suggests any differences in clinical severity between the subgroups. Therefore, it is important to implement measures effective at preventing disease due to both RSV A and RSV B to ensure impactful public health interventions. Monitoring overtime will be needed to assess the impact of waning antibody levels on subgroup-specific efficacy.
Keywords: Clinical severity; Epidemiology; Monoclonal antibodies; RSV subgroups; Respiratory syncytial virus; Vaccines.
© 2024. Pfizer.
Conflict of interest statement
Juliette Moyersoen, Marc Baay, Zuleika Aponte-Torres, and Hilde Vroling are employees of P95, which received funding from Pfizer in connection with the development of this manuscript. JM owns shares in the GSK group of companies as part of her previous employee remuneration. Charles Nuttens, Daniel Curcio, Bradford Gessner and Elizabeth Begier are employees of Pfizer and may own Pfizer stock.
Figures
References
-
- Public Health England. Respiratory syncytial virus. The Green Book; 2015.
-
- GBD 2016 Lower Respiratory Infections Collaborators. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect Dis. 2018;18(11):1191–210. - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

