Uncovering the unique characteristics of different groups of 5-HT5AR ligands with reference to their interaction with the target protein
- PMID: 38971919
- PMCID: PMC11387456
- DOI: 10.1007/s43440-024-00622-4
Uncovering the unique characteristics of different groups of 5-HT5AR ligands with reference to their interaction with the target protein
Abstract
Background: The serotonin 5-HT5A receptor has attracted much more research attention, due to the therapeutic potential of its ligands being increasingly recognized, and the possibilities that lie ahead of these findings. There is a growing body of evidence indicating that these ligands have procognitive, pro-social, and anti-depressant properties, which offers new avenues for the development of treatments that could address socially important conditions related to the malfunctioning of the central nervous system. The aim of our study was to unravel the molecular determinants for 5-HT5AR ligands that govern their activity towards the receptor.
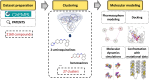
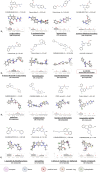
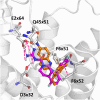
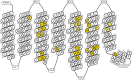
Methods: In response to the need for identification of molecular determinants for 5-HT5AR activity, we prepared a comprehensive collection of 5-HT5AR ligands, carefully gathering literature and patent data. Leveraging molecular modeling techniques, such as pharmacophore hypothesis development, docking, and molecular dynamics simulations enables to gain valuable insights into the specific interactions of 5-HT5AR ligand groups with the receptor.
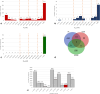
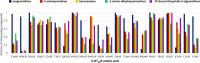
Results: The obtained comprehensive set of 2160 compounds was divided into dozens of subsets, and a pharmacophore model was developed for each group. The results from the docking and molecular dynamics simulations have enabled the identification of crucial ligand-protein interactions that are essential for the compound's activity towards 5-HT5AR.
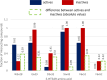
Conclusions: The findings from the molecular modeling study provide valuable insights that can guide medicinal chemists in the development of new 5-HT5AR ligands. Considering the pharmacological significance of these compounds, they have the potential to become impactful treatments for individuals and communities in the future. Understanding how different crystal/cryo-EM structures of 5-HT5AR affect molecular modeling experiments could have major implications for future computational studies on this receptor.
Keywords: 5-HT5AR; Docking; Ligand database; Molecular dynamics; Pharmacophore modeling; Serotonin receptors.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no conflict of interest.
Figures

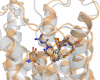



Similar articles
-
Structure-Based Design of a Chemical Probe Set for the 5-HT5A Serotonin Receptor.J Med Chem. 2022 Mar 10;65(5):4201-4217. doi: 10.1021/acs.jmedchem.1c02031. Epub 2022 Feb 23. J Med Chem. 2022. PMID: 35195401 Free PMC article.
-
Inactive and active state structures template selective tools for the human 5-HT5A receptor.Nat Struct Mol Biol. 2022 Jul;29(7):677-687. doi: 10.1038/s41594-022-00796-6. Epub 2022 Jul 14. Nat Struct Mol Biol. 2022. PMID: 35835867 Free PMC article.
-
Unveiling the potential of phytochemicals to inhibit nuclear receptor binding SET domain protein 2 for cancer: Pharmacophore screening, molecular docking, ADME properties, and molecular dynamics simulation investigations.PLoS One. 2024 Aug 20;19(8):e0308913. doi: 10.1371/journal.pone.0308913. eCollection 2024. PLoS One. 2024. PMID: 39163297 Free PMC article.
-
Molecular modeling approaches for the discovery of adenosine A2B receptor antagonists: current status and future perspectives.Drug Discov Today. 2019 Sep;24(9):1854-1864. doi: 10.1016/j.drudis.2019.05.011. Epub 2019 May 17. Drug Discov Today. 2019. PMID: 31103731 Review.
-
Structure-activity relationships of serotonin 5-HT7 receptors ligands: A review.Eur J Med Chem. 2019 Dec 1;183:111705. doi: 10.1016/j.ejmech.2019.111705. Epub 2019 Sep 16. Eur J Med Chem. 2019. PMID: 31581003 Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
