PARP inhibition with rucaparib alone followed by combination with atezolizumab: Phase Ib COUPLET clinical study in advanced gynaecological and triple-negative breast cancers
- PMID: 38971950
- PMCID: PMC11369183
- DOI: 10.1038/s41416-024-02776-7
PARP inhibition with rucaparib alone followed by combination with atezolizumab: Phase Ib COUPLET clinical study in advanced gynaecological and triple-negative breast cancers
Abstract
Background: Combining PARP inhibitors (PARPis) with immune checkpoint inhibitors may improve clinical outcomes in selected cancers. We evaluated rucaparib and atezolizumab in advanced gynaecological or triple-negative breast cancer (TNBC).
Methods: After identifying the recommended dose, patients with PARPi-naive BRCA-mutated or homologous recombination-deficient/loss-of-heterozygosity-high platinum-sensitive ovarian cancer or TNBC received rucaparib plus atezolizumab. Tumour biopsies were collected pre-treatment, during single-agent rucaparib run-in, and after starting combination therapy.
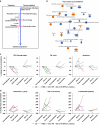
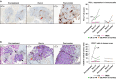
Results: The most common adverse events with rucaparib 600 mg twice daily and atezolizumab 1200 mg on Day 1 every 3 weeks were gastrointestinal effects, fatigue, liver enzyme elevations, and anaemia. Responding patients typically had BRCA-mutated tumours and higher pre-treatment tumour levels of PD-L1 and CD8 + T cells. Markers of DNA damage repair decreased during rucaparib run-in and combination treatment in responders, but typically increased in non-responders. Apoptosis signature expression showed the reverse. CD8 + T-cell activity and STING pathway activation increased during rucaparib run-in, increasing further with atezolizumab.
Conclusions: In this small study, rucaparib plus atezolizumab demonstrated acceptable safety and activity in BRCA-mutated tumours. Increasing anti-tumour immunity and inflammation might be a key mechanism of action for clinical benefit from the combination, potentially guiding more targeted development of such regimens.
Clinical trial registration: ClinicalTrials.gov (NCT03101280).
© 2024. The Author(s).
Conflict of interest statement
RK: clinical trial funding (to her institution) from Clovis Oncology; clinical trial grants from Merck Sharp & Dohme; consultancy for Pharmaceutica and Shattuck Pharma; honoraria from Clovis Oncology, AstraZeneca, GlaxoSmithKline, and Incyte; travel support from AstraZeneca, GlaxoSmithKline, and Sierra Oncology; and participation on data safety monitoring boards or advisory boards for Clovis Oncology, AstraZeneca, BeiGene, Eisai, GlaxoSmithKline, Incyte, iTeos Therapeutics, PharmaMar and Roche. AL: fees (to her institution) for advisory boards and educational presentations from AstraZeneca, Clovis, MSD, and GSK and a grant for translational research from AstraZeneca; she is the principal investigator on trials funded by AstraZeneca, MSD, and GSK. AO: consulting/advisory roles for Roche, AstraZeneca, PharmaMar, Clovis Oncology, Tesaro, ImmunoGen, Genmab, Mersana, GSK, Deciphera, AGENUS, Corcept Therapeutics, Eisai, EMD Serono, Medison, Merck Sharp & Dohme, Novocure, prIME Oncology, Shattuck Labs, Sutro Biopharma, ITeos Therapeutics, and Amgen; research funding (to her institution) from AbbVie, Abililty Pharmaceuticals, Advaxis, Aeterna Zentaris, Aprea Therapeutics, Clovis Oncology, Eisai, Roche, Regeneron, Agenus, AstraZeneca, BeiGene, Belgian Gynaecological Oncology Group (BGOG), BMS, Corcept Therapeutics, ImmunoGen, Iovance Biotherapeutics, Lilly, MedImmune, Merck, Merck Sharp & Dohme, Mundipharma Research, Novartis FarmacÃutica, Seagen, Seattle Genetics, Sutro Biopharma, Tesaro, and Verastem; and travel/accommodation/expenses from AstraZeneca, Clovis Oncology, PharmaMar, and Roche. AR: institutional grants from Eisai, PharmaMar, and Roche; honoraria from AstraZeneca, MSD, Clovis, GSK, PharmaMar, and Eisai; advisory board roles at AstraZeneca, MSD, Clovis, GSK, PharmaMar, and Eisai; and travel support from AstraZeneca, GSK, and PharmaMar. AG: honoraria from AstraZeneca, MSD, GSK, Clovis, and Roche. SC, CSS, LMo: Genentech employees and Roche shareholders. AS, ML-H: Roche employees and shareholders. JW: employee of Roche Products, Ltd, and a Roche shareholder. JX, KKL: former employees of Clovis Oncology. YG: former employee of Genentech. IR-C: honoraria from Abbvie, Advaxis, Amgen, AstraZeneca, Bristol Myers Squibb, Clovis Oncology, Deciphera, Genmab, GlaxoSmithKline, MERSANA, MSD Oncology, OxOnc, Pfizer, PharmaMar, Roche, and Tesaro; consulting/advisory roles for Abbvie, AstraZeneca, Bristol Myers Squibb, Clovis Oncology, Deciphera, Genmab, GlaxoSmithKline, Mersana, MSD Oncology, Pfizer, PharmaMar, Roche, and Tesaro; research funding from BMS and MSD Oncology; and travel/accommodation/expenses from Advaxis, AstraZeneca, BMS, Clovis Oncology, GlaxoSmithKline, PharmaMar, Roche, and Tesaro. LMi declares no competing interests.
Figures

References
-
- Pujade-Lauraine E, Ledermann JA, Selle F, Gebski V, Penson RT, Oza AM, et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18:1274–84. 10.1016/S1470-2045(17)30469-2 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

