Reasons for non-feasibility of therapeutic drug monitoring of oral targeted therapies in oncology - an analysis of the closed cohorts of a multicentre prospective study
- PMID: 38971952
- PMCID: PMC11369282
- DOI: 10.1038/s41416-024-02789-2
Reasons for non-feasibility of therapeutic drug monitoring of oral targeted therapies in oncology - an analysis of the closed cohorts of a multicentre prospective study
Abstract
Background: Therapeutic drug monitoring (TDM) - performing dose adjustments based on measured drug levels and established pharmacokinetic (PK) targets - could optimise treatment with drugs that show large interpatient variability in exposure. We evaluated the feasibility of TDM for multiple oral targeted therapies. Here we report on drugs for which routine TDM is not feasible.
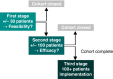
Methods: We evaluated drug cohorts from the Dutch Pharmacology Oncology Group - TDM study. Based on PK levels taken at pre-specified time points, PK-guided interventions were performed. Feasibility of TDM was evaluated, and based on the success and practicability of TDM, cohorts could be closed.
Results: For 10 out of 24 cohorts TDM was not feasible and inclusion was closed. A high incidence of adverse events resulted in closing the cabozantinib, dabrafenib/trametinib, everolimus, regorafenib and vismodegib cohort. The enzalutamide and erlotinib cohorts were closed because almost all PK levels were above target. Other, non-pharmacological reasons led to closing the palbociclib, olaparib and tamoxifen cohort.
Conclusions: Although TDM could help personalising treatment for many drugs, the above-mentioned reasons can influence its feasibility, usefulness and clinical applicability. Therefore, routine TDM is not advised for cabozantinib, dabrafenib/trametinib, enzalutamide, erlotinib, everolimus, regorafenib and vismodegib. Nonetheless, TDM remains valuable for individual clinical decisions.
© 2024. The Author(s).
Conflict of interest statement
MBAvdK, NADG, MM, KW, ELG, RFB, SLG, RAGvE, ALTI, AJEV, HMO, H-BFW, FJEL, IMED, DJARM, DJT, SLWK and ADRH declare that they do not have any financial or personal conflicting interests. HG reports institutional research funding from Daiichi Sankyo, Deciphera Pharmaceuticals, Novartis, Boehringer Ingelheim, AmMax Bio, Debiopharm, Cytovation; all outside the submitted work. AKLR reports institutional research funding from Merck and Tesaro; all outside the submitted work. NPvE reports research funding paid to the institute from Astellas, Ipsen; all outside the submitted work. RHJM reports research funding paid to the institute from Astellas, Bayer, Boehringer-Ingelheim, Cristal Therapeutics, Deuter Oncology, Nordic Pharma, Novartis, Pamgene, Pfizer, Roche, Sanofi, and Servier; all outside the submitted work. NS reports research grants paid to the institute from Abbvie, Actuate Therapeutics, Amgen, Array, Ascendis Pharma, AstraZeneca, Bayer, Blueprint Medicines, Boehringer Ingelheim, BridgeBio, Bristol-Myers Squibb, Cantargia, CellCentric, Cogent Biosciences, Cresecendo Biologics, Cytovation, Deciphera, Dragonfly, Eli Lilly, Exelixis, Genentech, GlaxoSmithKline, IDRx, Immunocore, Incyte, InteRNA, Janssen, Kinnate Biopharma, Kling Biotherapeutics, Luszana, Merck, Merck Sharp & Dohme, Merus, Molecular Partners, Navire Pharma, Novartis, Numab Therapeutics, Pfizer, Relay Pharmaceuticals, Revolution Medicin, Roche, Sanofi, Seattle Genetics, Taiho, Takeda; all outside the submitted work. NS provided consultation or attended advisory boards for Boehringer Ingelheim, Cogent Biosciences, Ellipses Pharma, Incyte, Luszana.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

