Piperine inhibits the proliferation of colorectal adenocarcinoma by regulating ARL3- mediated endoplasmic reticulum stress
- PMID: 38972051
- PMCID: PMC11734826
- DOI: 10.17305/bb.2024.10525
Piperine inhibits the proliferation of colorectal adenocarcinoma by regulating ARL3- mediated endoplasmic reticulum stress
Abstract
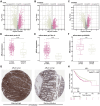
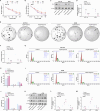
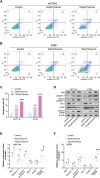
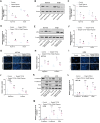
Colorectal adenocarcinoma (COAD) is a significant cause of cancer-related mortality worldwide, necessitating the identification of novel therapeutic targets and treatments. This research aimed to investigate the role of ARL3 in COAD progression and to explore the effects of Piperine on ARL3 expression, cell proliferation, epithelial-mesenchymal transition (EMT), and endoplasmic reticulum (ER) stress. Bioinformatics analysis of The Cancer Genome Atlas (TCGA)-COAD, GSE39582, and GSE44861 datasets assessed ARL3 expression levels. Immunohistochemical data from the Human Protein Atlas (HPA) database confirmed ARL3 overexpression in COAD. The association of ARL3 with COAD clinical parameters and prognosis was also examined. COAD cells were treated with Piperine, and in vitro assays evaluated cell proliferation, apoptosis, EMT marker expression, and ER stress (ERS) responses. ARL3 overexpression in COAD correlated with poor prognosis and varied across pathological stages. Piperine treatment inhibited COAD cell proliferation in a concentration- and time-dependent manner, as indicated by reduced Ki-67 levels and decreased colony-forming ability. Piperine induced S-phase cell cycle arrest and facilitated apoptosis in COAD cells, evidenced by changes in Bax, Bcl-2, cleaved caspase-3, and cleaved Poly (ADP-ribose) polymerase (PARP) levels. Moreover, Piperine downregulated ARL3 expression in COAD cells, thereby suppressing transforming growth factor beta (TGF-β)-induced EMT. Additionally, Piperine attenuated the ARL3-mediated ER stress response, significantly reducing binding immunoglobulin protein (BiP), inositol-requiring enzyme 1 alpha (p-IRE1α), activating transcription factor 6 (ATF6), and C/EBP homologous protein (CHOP) levels. Piperine exerts anti-cancer effects in COAD by modulating ARL3 expression, disrupting cell cycle progression, inhibiting the EMT pathway, and regulating ERS. These findings suggest that Piperine holds promise as a therapeutic agent for COAD through its targeting of ARL3.
Conflict of interest statement
Conflicts of interest: Authors declare no conflicts of interest.
Figures


References
-
- Pang B, Xu X, Lu Y, Jin H, Yang R, Jiang C, et al. Prediction of new targets and mechanisms for quercetin in the treatment of pancreatic cancer, colon cancer, and rectal cancer. Food Funct. 2019;10(9):5339–49. https://doi.org/10.1039/C9FO01168D. - PubMed
-
- Sharma N, Alam MS, Sharma A, Garg S, Maity MK. Colorectal cancer in young adults: epidemiology, risk factors, development, symptoms, traditional herbal therapy and prevention. J Pharm Negat Results. 2022;14:1370–82. https://doi.org/10.47750/pnr.2023.14.S02.167.
-
- Faezah S, Zaidi Z, Siti Rahmah M, Asma H, Syed Hassan SA, Nizam M, et al. Association of risk factors in tendency of colorectal cancer. Surg Chron. 2022;27(1):54–9.
-
- Cen X, Huang Y, Lu Z, Shao W, Zhuo C, Bao C, et al. LncRNA IGFL2-AS1 promotes the proliferation, migration, and invasion of colon cancer cells and is associated with patient prognosis. Cancer Manag Res. 2021;2021:5957–68. https://doi.org/10.2147/CMAR.S313775. - PMC - PubMed
-
- Gupta K, Jones JC, Farias VDA, Mackeyev Y, Singh PK, Quiñones-Hinojosa A, et al. Identification of synergistic drug combinations to target KRAS-driven chemoradioresistant cancers utilizing tumoroid models of colorectal adenocarcinoma and recurrent glioblastoma. Front Oncol. 2022;12:840241. https://doi.org/10.3389/fonc.2022.840241. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

