Excitatory synaptic structural abnormalities produced by templated aggregation of α-syn in the basolateral amygdala
- PMID: 38972360
- PMCID: PMC11632701
- DOI: 10.1016/j.nbd.2024.106595
Excitatory synaptic structural abnormalities produced by templated aggregation of α-syn in the basolateral amygdala
Erratum in
-
Corrigendum to "Excitatory synaptic structural abnormalities produced by templated aggregation of α-syn in the basolateral amygdala" [Neurobiology of Disease 199 (2024) 106595].Neurobiol Dis. 2025 Jan;204:106757. doi: 10.1016/j.nbd.2024.106757. Epub 2025 Jan 6. Neurobiol Dis. 2025. PMID: 39779046 No abstract available.
Abstract
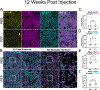
Parkinson's disease (PD) and Dementia with Lewy bodies (DLB) are characterized by neuronal α-synuclein (α-syn) inclusions termed Lewy Pathology, which are abundant in the amygdala. The basolateral amygdala (BLA), in particular, receives projections from the thalamus and cortex. These projections play a role in cognition and emotional processing, behaviors which are impaired in α-synucleinopathies. To understand if and how pathologic α-syn impacts the BLA requires animal models of α-syn aggregation. Injection of α-syn pre-formed fibrils (PFFs) into the striatum induces robust α-syn aggregation in excitatory neurons in the BLA that corresponds with reduced contextual fear conditioning. At early time points after aggregate formation, cortico-amygdala excitatory transmission is abolished. The goal of this project was to determine if α-syn inclusions in the BLA induce synaptic degeneration and/or morphological changes. In this study, we used C57BL/6 J mice injected bilaterally with PFFs in the dorsal striatum to induce α-syn aggregate formation in the BLA. A method was developed using immunofluorescence and three-dimensional reconstruction to analyze excitatory cortico-amygdala and thalamo-amygdala presynaptic terminals closely juxtaposed to postsynaptic densities. The abundance and morphology of synapses were analyzed at 6- or 12-weeks post-injection of PFFs. α-Syn aggregate formation in the BLA did not cause a significant loss of synapses, but cortico-amygdala and thalamo-amygdala presynaptic terminals and postsynaptic densities with aggregates of α-syn show increased volumes, similar to previous findings in human DLB cortex, and in non-human primate models of PD. Transmission electron microscopy showed that asymmetric synapses in mice with PFF-induced α-syn aggregates have reduced synaptic vesicle intervesicular distances, similar to a recent study showing phospho-serine-129 α-syn increases synaptic vesicle clustering. Thus, pathologic α-syn causes major alterations to synaptic architecture in the BLA, potentially contributing to behavioral impairment and amygdala dysfunction observed in synucleinopathies.
Keywords: Basolateral amygdala; Dementia with Lewy bodies; Glutamatergic; P-α-syn; Parkinson's disease; Presynaptic terminal; Synapses.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Figures






Update of
-
Cortico-amygdala synaptic structural abnormalities produced by templated aggregation of α-synuclein.bioRxiv [Preprint]. 2024 May 15:2024.05.15.594419. doi: 10.1101/2024.05.15.594419. bioRxiv. 2024. Update in: Neurobiol Dis. 2024 Sep;199:106595. doi: 10.1016/j.nbd.2024.106595. PMID: 38798467 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

