Efficacy of Chemotherapy-Free Regimens in the Treatment of Philadelphia Chromosome-Positive Acute Lymphoblastic Leukemia: A Systematic Review and Meta-Analysis
- PMID: 38972767
- PMCID: PMC11809103
- DOI: 10.1016/j.clml.2024.06.002
Efficacy of Chemotherapy-Free Regimens in the Treatment of Philadelphia Chromosome-Positive Acute Lymphoblastic Leukemia: A Systematic Review and Meta-Analysis
Abstract
Introduction: The historical standard of care for Ph+ ALL is chemotherapy plus a tyrosine kinase inhibitor (TKI). Recently chemotherapy-free regimens have shown promising efficacy. We performed a meta-analysis to compare the efficacy of chemotherapy-free regimens for Ph+ ALL.
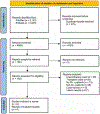
Methods: We searched PubMed and Embase for chemotherapy-free regimens for Ph+ ALL published between January 2000 and October 2023. Of the 5,348 articles screened, 9 nonrandomized clinical trials enrolling 413 patients were included. Two trials (N = 117) included treatment with 3 agents (blinatumomab, TKI, and steroid) and 7 trials (N = 248) included treatment with 2 agents (TKI and steroids). R software was used to conduct the meta-analysis (PROSPERO registration no. CRD42023482439).
Results: The pooled complete molecular response (CMR) rate of patients receiving a TKI, blinatumomab, and steroids was 81% (95%CI, 69%-89%). TKIs plus blinatumomab were nearly 6 times as likely to have CMR (odds ratio [OR], 5.98; 95%CI, 2.99-11.96) and more than 5 times as likely to be alive at 1-year (OR, 5.1; 95%CI, 1.74-14.9) as compared to TKIs alone. Patients receiving ponatinib were about twice as likely as those receiving dasatinib to achieve CMR (OR, 2.51; 95%CI, 0.72-8.72).
Conclusion: Adding blinatumomab to TKIs and steroids significantly improved Ph+ ALL patients' response and survival rates. Regimens with ponatinib elicited higher molecular response rates than those with other TKIs. The high response and survival rates achieved with blinatumomab plus TKIs and steroids suggest that further studies are required to assess the need for intensive treatments such as chemotherapy or stem cell transplant in these patients.
Keywords: Blinatumomab; Tyrosine kinase inhibitors.
Copyright © 2024 Elsevier Inc. All rights reserved.
Conflict of interest statement
Disclosures H.K.: research funding from AbbVie, Amgen, Ascentage, BMS, Daiichi-Sankyo, Immunogen, Jazz, Novartis; and honoraria/advisory board/consultancy from AbbVie, Amgen, Amphista, Ascentage, Astellas, Biologix, Curis, Ipsen Biopharmaceuticals, KAHR Medical, Labcorp, Novartis, Pfizer, Shenzhen Target Rx, Stemline, Takeda and Taiho Pharmaceutical Canada. E.J. received research grants from Abbvie, Adaptive Biotechnologies, Amgen, Pfizer, and Takeda; and consultancy fees from Abbvie, Adaptive Biotechnologies, Amgen, BMS, Genentech, Incyte, Novartis, Pfizer, and Takeda. F.R.: research funding from Amgen, Astex, Pharmaceuticals/Taiho Oncology, BMS/Celgene, Syos, AbbVie, Prelude, Xencor, Astellas Pharma, and Biomea Fusion as well as honoraria from Amgen, BMS/Celgene, Syos, AbbVie, and Astellas Pharma; has been a board of directors or advisory committee member for Astex Pharmaceuticals/Taiho Oncology; and has been a consultant for BMS/Celgene, Syos, Novartis, AbbVie, AstraZeneca, and Astellas Pharma. N.J.: served as a consultant/advisory role with Pharmacyclics, Novartis, ADC Therapeutics, Pfizer, Servier, Novimmune, and Adaptive Biotechnologies. He has received institutional research funding from Pharmacyclics, Genentech, Abbvie, Pfizer, Incyte, BMS, Infinity, ADC Therapeutics, Seattle Genetics, Celgene, and Servier. N.J.S.: has been a consultant for Takeda Oncology, AstraZeneca, Amgen, Novartis, and Pfizer; received research funding from Takeda Oncology, Astellas, and Stemline Therapeutics; and received honoraria from Amgen K.S.: received research funding from Novartis, honoraria from Otsuka, and consultation fees from Daiichi-Sankyo, Novartis, and Pfizer. M.A.A. and W.A. declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources