Inhibition of PKC-δ retards kidney fibrosis via inhibiting cGAS-STING signaling pathway in mice
- PMID: 38972937
- PMCID: PMC11228024
- DOI: 10.1038/s41420-024-02087-z
Inhibition of PKC-δ retards kidney fibrosis via inhibiting cGAS-STING signaling pathway in mice
Abstract
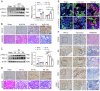
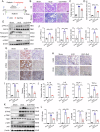
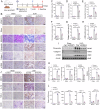
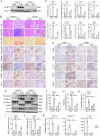
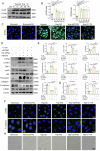
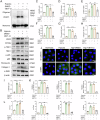
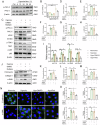
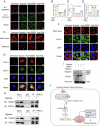
Kidney fibrosis is considered to be the ultimate aggregation pathway of chronic kidney disease (CKD), but its underlying mechanism remains elusive. Protein kinase C-delta (PKC-δ) plays critical roles in the control of growth, differentiation, and apoptosis. In this study, we found that PKC-δ was highly upregulated in human biopsy samples and mouse kidneys with fibrosis. Rottlerin, a PKC-δ inhibitor, alleviated unilateral ureteral ligation (UUO)-induced kidney fibrosis, inflammation, VDAC1 expression, and cGAS-STING signaling pathway activation. Adeno-associated virus 9 (AAV9)-mediated VDAC1 silencing or VBIT-12, a VDAC1 inhibitor, attenuated renal injury, inflammation, and activation of cGAS-STING signaling pathway in UUO mouse model. Genetic and pharmacologic inhibition of STING relieved renal fibrosis and inflammation in UUO mice. In vitro, hypoxia resulted in PKC-δ phosphorylation, VDAC1 oligomerization, and activation of cGAS-STING signaling pathway in HK-2 cells. Inhibition of PKC-δ, VDAC1 or STING alleviated hypoxia-induced fibrotic and inflammatory responses in HK-2 cells, respectively. Mechanistically, PKC-δ activation induced mitochondrial membrane VDAC1 oligomerization via direct binding VDAC1, followed by the mitochondrial DNA (mtDNA) release into the cytoplasm, and subsequent activated cGAS-STING signaling pathway, which contributed to the inflammation leading to fibrosis. In conclusion, this study has indicated for the first time that PKC-δ is an important regulator in kidney fibrosis by promoting cGAS-STING signaling pathway which mediated by VDAC1. PKC-δ may be useful for treating renal fibrosis and subsequent CKD.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

