Comparative immune profiling of pancreatic ductal adenocarcinoma progression among South African patients
- PMID: 38973003
- PMCID: PMC11229237
- DOI: 10.1186/s12885-024-12595-x
Comparative immune profiling of pancreatic ductal adenocarcinoma progression among South African patients
Abstract
Background: Pancreatic Ductal Adenocarcinoma (PDAC) is an aggressive cancer characterized by an immunosuppressive microenvironment. Patients from specific ethnicities and population groups have poorer prognoses than others. Therefore, a better understanding of the immune landscape in such groups is necessary for disease elucidation, predicting patient outcomes and therapeutic targeting. This study investigated the expression of circulating key immune cell markers in South African PDAC patients of African ancestry.
Methods: Blood samples were obtained from a total of 6 healthy volunteers (HC), 6 Chronic Pancreatitis (CP) and 34 PDAC patients consisting of 22 resectable (RPC), 8 locally advanced (LAPC) and 4 metastatic (MPC). Real-time Quantitative Polymerase Chain reactions (RT-qPCR), Metabolomics, Enzyme-Linked Immunosorbent Assay (ELISA), Reactive Oxygen Species (ROS), and Immunophenotyping assays were conducted. Statistical analysis was conducted in R (v 4.3.2). Additional analysis of single-cell RNA data from 20 patients (16 PDAC and 4 controls) was conducted to interrogate the distribution of T-cell and Natural Killer cell populations.
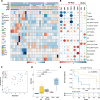
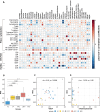
Results: Granulocyte and neutrophil levels were significantly elevated while lymphocytes decreased with PDAC severity. The total percentages of CD3 T-cell subpopulations (helper and double negative T-cells) decreased when compared to HC. Although both NK (p = 0.014) and NKT (p < 0.001) cell levels increased as the disease progressed, their subsets: NK CD56dimCD16- (p = 0.024) and NKTs CD56+ (p = 0.008) cell levels reduced significantly. Of note is the negative association of NK CD56dimCD16- (p < 0.001) cell levels with survival time. The gene expression analyses showed no statistically significant correlation when comparing the PDAC groups with the controls. The inflammatory status of PDAC was assessed by ROS levels of serum which were elevated in CP (p = 0.025), (RPC (p = 0.003) and LAPC (p = 0.008)) while no significant change was observed in MPC, compared to the HC group. ROS was shown to be positively correlated with GlycA (R = 0.45, p = 0.0096). Single-cell analyses showed a significant difference in the ratio of NKT cells per total cell counts in LAPC (p < 0.001) and MPC (p < 0.001) groups compared with HC, confirming observations in our sample group.
Conclusion: The expression of these immune cell markers observed in this pilot study provides insight into their potential roles in tumour progression in the patient group and suggests their potential utility in the development of immunotherapeutic strategies.
Keywords: CD4; CD8; Immune cells; Immunosuppression; PDAC; Pancreatic ductal adenocarcinoma.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

