Distinct Thalamo-Subcortical Circuits Underlie Painful Behavior and Depression-Like Behavior Following Nerve Injury
- PMID: 38973158
- PMCID: PMC11425852
- DOI: 10.1002/advs.202401855
Distinct Thalamo-Subcortical Circuits Underlie Painful Behavior and Depression-Like Behavior Following Nerve Injury
Abstract
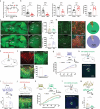
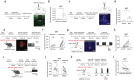
Clinically, chronic pain and depression often coexist in multiple diseases and reciprocally reinforce each other, which greatly escalates the difficulty of treatment. The neural circuit mechanism underlying the chronic pain/depression comorbidity remains unclear. The present study reports that two distinct subregions in the paraventricular thalamus (PVT) play different roles in this pathological process. In the first subregion PVT posterior (PVP), glutamatergic neurons (PVPGlu) send signals to GABAergic neurons (VLPAGGABA) in the ventrolateral periaqueductal gray (VLPAG), which mediates painful behavior in comorbidity. Meanwhile, in another subregion PVT anterior (PVA), glutamatergic neurons (PVAGlu) send signals to the nucleus accumbens D1-positive neurons and D2-positive neurons (NAcD1→D2), which is involved in depression-like behavior in comorbidity. This study demonstrates that the distinct thalamo-subcortical circuits PVPGlu→VLPAGGABA and PVAGlu→NAcD1→D2 mediated painful behavior and depression-like behavior following spared nerve injury (SNI), respectively, which provides the circuit-based potential targets for preventing and treating comorbidity.
Keywords: chronic pain; comorbidity; depression; nucleus accumbens; paraventricular thalamus anterior; paraventricular thalamus posterior; ventrolateral periaqueductal gray.
© 2024 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- a) Roughan W. H., Campos A. I., Garcia‐Marin L. M., Cuellar‐Partida G., Lupton M. K., Hickie I. B., Medland S. E., Wray N. R., Byrne E. M., Ngo T. T., Martin N. G., Renteria M. E., Front. Psychiatry 2021, 12, 643609; - PMC - PubMed
- b) Dhanju S., Kennedy S. H., Abbey S., Katz J., Weinrib A., Clarke H., Bhat V., Ladha K., Scand J. Pain 2019, 19, 319. - PubMed
-
- Yen C. T., Lu P. L., Acta Anaesthesiol Taiwan 2013, 51, 73. - PubMed
MeSH terms
Grants and funding
- 31970936/National Natural Science Foundation of China
- 82201377/National Natural Science Foundation of China
- 81771144/National Natural Science Foundation of China
- 81870878/National Natural Science Foundation of China
- 2023B0303010002/Key-Area Research, Development Program of Guangdong Province
- Guangdong Basic
- 2022A1515012124/Applied Basic Research Foundation
- 2022A1515012259/Applied Basic Research Foundation
- 2023A1515030020/Applied Basic Research Foundation
- 2021A1515011134/Applied Basic Research Foundation
- 2019B151502010/Applied Basic Research Foundation
- 2022B1515120026/Applied Basic Research Foundation
- 2019A1515110164/Applied Basic Research Foundation
- 2023A1515010241/Guangdong Province Natural Science Fund
- Guangzhou Science
- 20226060004/Technology Plan Project
- 2023A03J0408/Technology Plan Project
- 2023A03J0574/Technology Plan Project
- 202201010988/Applied Basic Research Project of Guangzhou
- SL2023A04J00650/Applied Basic Research Project of Guangzhou
- WYYXQN-2021004/Excellent Young Researchers Program of the 5th Affiliated Hospital of SYSU
- 2021B1212040017/Science and Technology Planning Project of Guangdong Province
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
