Hypoxia-Preconditioned BMSC-Derived Exosomes Induce Mitophagy via the BNIP3-ANAX2 Axis to Alleviate Intervertebral Disc Degeneration
- PMID: 38973294
- PMCID: PMC11425632
- DOI: 10.1002/advs.202404275
Hypoxia-Preconditioned BMSC-Derived Exosomes Induce Mitophagy via the BNIP3-ANAX2 Axis to Alleviate Intervertebral Disc Degeneration
Abstract
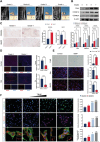
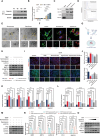
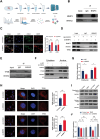
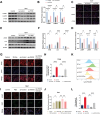
Intervertebral disc degeneration (IVDD) is a chronic degenerative disease involving the aging and loss of proliferative capacity of nucleus pulposus cells (NPCs), processes heavily dependent on mitochondrial dynamics and autophagic flux. This study finds that the absence of BCL2/adenovirus E1B 19 kDa interacting protein 3 (BNIP3) is associated with senescence-related NPC degeneration, disrupting mitochondrial quality control. Bone marrow mesenchymal stem cells (BMSCs) have multidirectional differentiation potential and produce extracellular vesicles containing cellular activators. Therefore, in this study, BMSCs are induced under hypoxic stimulation to deliver BNIP3-rich extracellular vesicles to NPCs, thereby alleviating aging-associated mitochondrial autophagic flux, promoting damaged mitochondrial clearance, and restoring mitochondrial quality control. Mechanistically, BNIP3 is shown to interact with the membrane-bound protein annexin A2 (ANXA2), enabling the liberation of the transcription factor EB (TFEB) from the ANXA2-TFEB complex, promoting TFEB nuclear translocation, and regulating autophagy and lysosomal gene activation. Furthermore, a rat model of IVDD is established and verified the in vivo efficacy of the exosomes in repairing disc injuries, delaying NPC aging, and promoting extracellular matrix (ECM) synthesis. In summary, hypoxia-induced BMSC exosomes deliver BNIP3-rich vesicles to alleviate disc degeneration by activating the mitochondrial BNIP3/ANXA2/TFEB axis, providing a new target for IVDD treatment.
Keywords: Intervertebral disc degeneration; bone marrow mesenchymal stem cells; exosomes; hypoxia‐preconditioned mesenchymal stem cells; matrix reconstruction; mitophagy.
© 2024 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- a) Cieza A., Causey K., Kamenov K., Hanson S. W., Chatterji S., Vos T., Lancet 2020, 396, P2006; - PMC - PubMed
- b) Hartvigsen J., Hancock M. J., Kongsted A., Louw Q., Ferreira M. L., Genevay S., Hoy D., Karppinen J., Pransky G., Sieper J., Smeets R. J., Underwood M., Lancet Low Back Pain Series Working Group Lancet, 2018, 391, 2356. - PubMed
-
- Knezevic N. N., Candido K. D., Vlaeyen J. W. S., Van Zundert J., Cohen S. P., Lancet 2021, 398, 78. - PubMed
-
- a) Chen S., Wu X., Lai Y., Chen D., Bai X., Liu S., Wu Y., Chen M., Lai Y., Cao H., Shao Z., Xiao G., Bone Res. 2022, 10, 5; - PMC - PubMed
- b) Romaniyanto, F. M. , Sigit Prakoeswa C. R., Notobroto H. B., Tinduh D., Ausrin R., Rantam F. A., Suroto H., Utomo D. N., Rhatomy S., Ann. Med. Surg. 2022, 77, 103619. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
