DCAF7 Acts as A Scaffold to Recruit USP10 for G3BP1 Deubiquitylation and Facilitates Chemoresistance and Metastasis in Nasopharyngeal Carcinoma
- PMID: 38973296
- PMCID: PMC11423104
- DOI: 10.1002/advs.202403262
DCAF7 Acts as A Scaffold to Recruit USP10 for G3BP1 Deubiquitylation and Facilitates Chemoresistance and Metastasis in Nasopharyngeal Carcinoma
Abstract
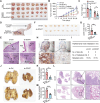
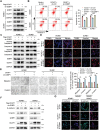
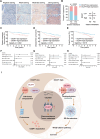
Despite docetaxel combined with cisplatin and 5-fluorouracil (TPF) being the established treatment for advanced nasopharyngeal carcinoma (NPC), there are patients who do not respond positively to this form of therapy. However, the mechanisms underlying this lack of benefit remain unclear. DCAF7 is identified as a chemoresistance gene attenuating the response to TPF therapy in NPC patients. DCAF7 promotes the cisplatin resistance and metastasis of NPC cells in vitro and in vivo. Mechanistically, DCAF7 serves as a scaffold protein that facilitates the interaction between USP10 and G3BP1, leading to the elimination of K48-linked ubiquitin moieties from Lys76 of G3BP1. This process helps prevent the degradation of G3BP1 via the ubiquitin‒proteasome pathway and promotes the formation of stress granule (SG)-like structures. Moreover, knockdown of G3BP1 successfully reversed the formation of SG-like structures and the oncogenic effects of DCAF7. Significantly, NPC patients with increased levels of DCAF7 showed a high risk of metastasis, and elevated DCAF7 levels are linked to an unfavorable prognosis. The study reveals DCAF7 as a crucial gene for cisplatin resistance and offers further understanding of how chemoresistance develops in NPC. The DCAF7-USP10-G3BP1 axis contains potential targets and biomarkers for NPC treatment.
Keywords: DCAF7; chemoresistance; chemotherapy; deubiquitylation; nasopharyngeal carcinoma.
© 2024 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- a) Zhang L., Chen Q. Y., Liu H., Tang L. Q., Mai H. Q., Drug Des. Devel. Ther. 2013, 7, 37; - PMC - PubMed
- b) Qiao H., Tan X. R., Li H., Li J. Y., Chen X. Z., Li Y. Q., Li W. F., Tang L. L., Zhou G. Q., Zhang Y., Liang Y. L., He Q. M., Zhao Y., Huang S. Y., Gong S., Li Q., Ye M. L., Chen K. L., Sun Y., Ma J., Liu N., JAMA Oncol. 2022, 8, 1301. - PMC - PubMed
-
- a) Tang L. L., Chen Y. P., Chen C. B., Chen M. Y., Chen N. Y., Chen X. Z., Du X. J., Fang W. F., Feng M., Gao J., Han F., He X., Hu C. S., Hu D. S., Hu G. Y., Jiang H., Jiang W., Jin F., Lang J. Y., Li J. G., Lin S. J., Liu X., Liu Q. F., Ma L., Mai H. Q., Qin J. Y., Shen L. F., Sun Y., Wang P. G., Wang R. S., et al., Cancer Commun. 2021, 41, 1195; - PMC - PubMed
- b) Sun Y., Li W. F., Chen N. Y., Zhang N., Hu G. Q., Xie F. Y., Sun Y., Chen X. Z., Li J. G., Zhu X. D., Hu C. S., Xu X. Y., Chen Y. Y., Hu W. H., Guo L., Mo H. Y., Chen L., Mao Y. P., Sun R., Ai P., Liang S. B., Long G. X., Zheng B. M., Feng X. L., Gong X. C., Li L., Shen C. Y., Xu J. Y., Guo Y., Chen Y. M., et al., Lancet Oncol. 2016, 17, 1509.
-
- Peng H., Chen L., Li W. F., Guo R., Mao Y. P., Zhang Y., Guo Y., Sun Y., Ma J., Cancer 2017, 123, 1643. - PubMed
-
- Faux M. C., Scott J. D., Cell 1996, 85, 9. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
