An Integrated DAIDS Laboratory Oversight Framework: Application of the DAIDS GCLP Guidelines
- PMID: 38973467
- PMCID: PMC11631790
- DOI: 10.1089/AID.2024.0041
An Integrated DAIDS Laboratory Oversight Framework: Application of the DAIDS GCLP Guidelines
Abstract
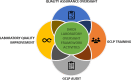
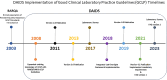
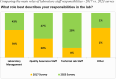
The Division of AIDS (DAIDS) Good Clinical Laboratory Practice (GCLP) Guidelines establish a framework to guide the oversight of laboratories supporting DAIDS-sponsored clinical research or trials. Compliance with these guidelines promotes data reliability, consistency, and validity, and the safety of the clinical research or trial participants and laboratory staff, as well as ensures adherence to regulatory requirements. This article describes the application of the DAIDS GCLP Guidelines, the DAIDS Integrated Laboratory Oversight Framework, and the coordinated efforts of the collaborative oversight team of laboratory experts to support and monitor the performance of over 175 participating laboratories worldwide. Data from two self-administered online surveys conducted in 2017 and 2023 assessed the laboratory staff's experience implementing the GCLP Guidelines. The results of the 2017 survey were instrumental in informing changes to GCLP audit activities and promoting harmonization in the approach to laboratory oversight. A key finding from the 2023 survey results is the preference for hybrid GCLP training, encompassing face-to-face and online modules. Overall, both surveys acknowledged satisfaction with applying and implementing GCLP Guidelines. The need to effectively disseminate information about DAIDS laboratory oversight requirements to support the improved implementation of GCLP Guidelines was notable from both survey results. The collaborative team of laboratory experts and the integrated oversight approach promote knowledge-sharing and accountability to support the application of the GCLP Guidelines and compliance monitoring. The systematic implementation of the integrated laboratory oversight activities helped identify valuable lessons for improving laboratory performance and opportunities to strengthen quality oversight for laboratories participating in clinical research or trials.
Keywords: Clinical Trials; DAIDS GCLP guidelines; GCLP audit; GCLP training; laboratory performance; quality assurance.
Figures


References
-
- Division of AIDS (DAIDS) Site Clinical Operations and Research Essentials (SCORE) Manual | NIH: National Institute of Allergy and Infectious Diseases. Available from: https://www.niaid.nih.gov/research/daids-score-manual
-
- Stiles T, Grant V, Mawbey N. Good clinical laboratory practice (GCLP): A quality system for laboratories that undertake the analysis of samples from clinical trials. British Association of Research Quality Assurance (BARQA). Suffolk, United Kingdom; 2003.
-
- National Institute of Allergy and Infectious Diseases. (NIAID) Division of AIDS (DAIDS): Good Clinical Laboratory Practice (GCLP) Guidelines. 2021.. Available from: https://www.niaid.nih.gov/sites/default/files/gclpstandards.pdf
MeSH terms
LinkOut - more resources
Full Text Sources
