IL-10 inhibition during immunization improves vaccine-induced protection against Staphylococcus aureus infection
- PMID: 38973612
- PMCID: PMC11383370
- DOI: 10.1172/jci.insight.178216
IL-10 inhibition during immunization improves vaccine-induced protection against Staphylococcus aureus infection
Abstract
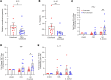
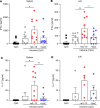
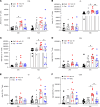
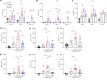
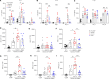
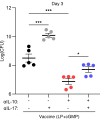
Staphylococcus aureus is a major human pathogen. An effective anti-S. aureus vaccine remains elusive as the correlates of protection are ill-defined. Targeting specific T cell populations is an important strategy for improving anti-S. aureus vaccine efficacy. Potential bottlenecks that remain are S. aureus-induced immunosuppression and the impact this might have on vaccine-induced immunity. S. aureus induces IL-10, which impedes effector T cell responses, facilitating persistence during both colonization and infection. Thus, it was hypothesized that transient targeting of IL-10 might represent an innovative way to improve vaccine efficacy. In this study, IL-10 expression was elevated in the nares of persistent carriers of S. aureus, and this was associated with reduced systemic S. aureus-specific Th1 responses. This suggests that systemic responses are remodeled because of commensal exposure to S. aureus, which negatively implicates vaccine function. To provide proof of concept that targeting immunosuppressive responses during immunization may be a useful approach to improve vaccine efficacy, we immunized mice with T cell-activating vaccines in combination with IL-10-neutralizing antibodies. Blocking IL-10 during vaccination enhanced effector T cell responses and improved bacterial clearance during subsequent systemic and subcutaneous infection. Taken together, these results reveal a potentially novel strategy for improving anti-S. aureus vaccine efficacy.
Keywords: Bacterial infections; Bacterial vaccines; Immunology; T cells; Vaccines.
Conflict of interest statement
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

