Neural pathways that compel us to scratch an itch
- PMID: 38973668
- PMCID: PMC7617712
Neural pathways that compel us to scratch an itch
Abstract
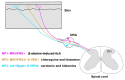
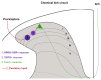
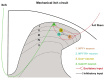
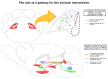
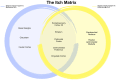
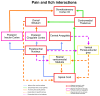
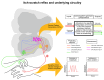
Itch is a unique sensory experience that is responded to by scratching. How pruritogens, which are mechanical and chemical stimuli with the potential to cause itch, engage specific pathways in the peripheral and central nervous system has been a topic of intense investigation over the last few years. Studies employing recently developed molecular, physiological, and behavioral techniques have delineated the dedicated mechanisms that transmit itch information to the brain. This review outlines the genetically defined and evolutionary conserved circuits for itch ranging from the skin-innervating peripheral neurons to the cortical neurons that drive scratching. Moreover, scratch suppression of itch is attributed to the concurrent activation of pain and itch pathways. Hence, we discuss the similarities between circuits driving pain and itch.
Conflict of interest statement
Figures
References
-
- Adebayo RA, Sofowora GG, Onayemi O, et al. Chloroquine-induced pruritus in malaria fever: contribution of malaria parasitaemia and the effects of prednisolone, niacin, and their combination, compared with antihistamine. Br J Clin Pharmacol. 1997;44:157–161. doi: 10.1046/j.1365-2125.1997.00612.x. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
