Near Infrared Biomimetic Hybrid Magnetic Nanocarrier for MRI-Guided Thermal Therapy
- PMID: 38973727
- PMCID: PMC11891835
- DOI: 10.1021/acsami.4c03434
Near Infrared Biomimetic Hybrid Magnetic Nanocarrier for MRI-Guided Thermal Therapy
Abstract
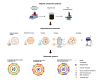
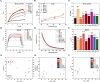
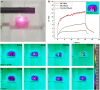
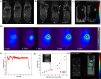
Cell-membrane hybrid nanoparticles (NPs) are designed to improve drug delivery, thermal therapy, and immunotherapy for several diseases. Here, we report the development of distinct biomimetic magnetic nanocarriers containing magnetic nanoparticles encapsulated in vesicles and IR780 near-infrared dyes incorporated in the membranes. Distinct cell membranes are investigated, red blood cell (RBC), melanoma (B16F10), and glioblastoma (GL261). Hybrid nanocarriers containing synthetic lipids and a cell membrane are designed. The biomedical applications of several systems are compared. The inorganic nanoparticle consisted of Mn-ferrite nanoparticles with a core diameter of 15 ± 4 nm. TEM images show many multicore nanostructures (∼40 nm), which correlate with the hydrodynamic size. Ultrahigh transverse relaxivity values are reported for the magnetic NPs, 746 mM-1s-1, decreasing respectively to 445 mM-1s-1 and 278 mM-1s-1 for the B16F10 and GL261 hybrid vesicles. The ratio of relaxivities r2/r1 decreased with the higher encapsulation of NPs and increased for the biomimetic liposomes. Therapeutic temperatures are achieved by both, magnetic nanoparticle hyperthermia and photothermal therapy. Photothermal conversion efficiency ∼25-30% are reported. Cell culture revealed lower wrapping times for the biomimetic vesicles. In vivo experiments with distinct routes of nanoparticle administration were investigated. Intratumoral injection proved the nanoparticle-mediated PTT efficiency. MRI and near-infrared images showed that the nanoparticles accumulate in the tumor after intravenous or intraperitoneal administration. Both routes benefit from MRI-guided PTT and demonstrate the multimodal theranostic applications for cancer therapy.
Keywords: SPION; cancer; cell membrane nanoparticles; glioblastoma.; thermal nanomedicine.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





Similar articles
-
Manganese doped iron oxide theranostic nanoparticles for combined T1 magnetic resonance imaging and photothermal therapy.ACS Appl Mater Interfaces. 2015 Mar 4;7(8):4650-8. doi: 10.1021/am5080453. Epub 2015 Feb 20. ACS Appl Mater Interfaces. 2015. PMID: 25672225
-
Polymer coated gold-ferric oxide superparamagnetic nanoparticles for theranostic applications.J Nanobiotechnology. 2018 Oct 13;16(1):80. doi: 10.1186/s12951-018-0405-7. J Nanobiotechnology. 2018. PMID: 30316298 Free PMC article.
-
Intrinsically Mn2+-Chelated Polydopamine Nanoparticles for Simultaneous Magnetic Resonance Imaging and Photothermal Ablation of Cancer Cells.ACS Appl Mater Interfaces. 2015 Aug 12;7(31):16946-52. doi: 10.1021/acsami.5b06265. Epub 2015 Aug 3. ACS Appl Mater Interfaces. 2015. PMID: 26196160
-
Recent Advances in Biomimetic Nanocarrier-Based Photothermal Therapy for Cancer Treatment.Int J Mol Sci. 2023 Oct 23;24(20):15484. doi: 10.3390/ijms242015484. Int J Mol Sci. 2023. PMID: 37895165 Free PMC article. Review.
-
Magnetic nanoparticles as contrast agents in biomedical imaging: recent advances in iron- and manganese-based magnetic nanoparticles.Drug Metab Rev. 2014 May;46(2):142-54. doi: 10.3109/03602532.2013.876429. Drug Metab Rev. 2014. PMID: 24754519 Review.
Cited by
-
Synthetic circRNA therapeutics: innovations, strategies, and future horizons.MedComm (2020). 2024 Nov 9;5(11):e720. doi: 10.1002/mco2.720. eCollection 2024 Nov. MedComm (2020). 2024. PMID: 39525953 Free PMC article. Review.
-
Selective Photothermal Eradication of Glioblastoma Cells Coexisting with Astrocytes by Anti-EGFR-Coated Raman Tags.ACS Appl Bio Mater. 2025 Apr 21;8(4):3119-3126. doi: 10.1021/acsabm.4c01986. Epub 2025 Mar 18. ACS Appl Bio Mater. 2025. PMID: 40100247 Free PMC article.
References
-
- Soares G.; Pinto L.; Liebl M.; Biasotti G.; Prospero A.; Stoppa E.; Bakuzis A.; Baffa O.; Wiekhorst F.; Miranda J. R. A. Quantitative Imaging of Magnetic Nanoparticles in an Unshielded Environment Using a large AC Susceptibility Array. Journal of Biological Engineering 2022, 16, 25.10.1186/s13036-022-00305-9. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

