Optimal Dynamic Regimes for CO Oxidation Discovered by Reinforcement Learning
- PMID: 38973853
- PMCID: PMC11223201
- DOI: 10.1021/acsomega.3c10422
Optimal Dynamic Regimes for CO Oxidation Discovered by Reinforcement Learning
Abstract
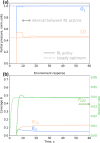
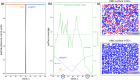
Metal nanoparticles are widely used as heterogeneous catalysts to activate adsorbed molecules and reduce the energy barrier of the reaction. Reaction product yield depends on the interplay between elementary processes: adsorption, activation, desorption, and reaction. These processes, in turn, depend on the inlet gas composition, temperature, and pressure. At a steady state, the active surface sites may be inaccessible due to adsorbed reagents. Periodic regime may thus improve the yield, but the appropriate period and waveform are not known in advance. Dynamic control should account for surface and atmospheric modifications and adjust reaction parameters according to the current state of the system and its history. In this work, we applied a reinforcement learning algorithm to control CO oxidation on a palladium catalyst. The policy gradient algorithm was trained in the theoretical environment, parametrized from experimental data. The algorithm learned to maximize the CO2 formation rate based on CO and O2 partial pressures for several successive time steps. Within a unified approach, we found optimal stationary, periodic, and nonperiodic regimes for different problem formulations and gained insight into why the dynamic regime can be preferential. In general, this work contributes to the task of popularizing the reinforcement learning approach in the field of catalytic science.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures







References
-
- Pareek V.; Bhargava A.; Gupta R.; Jain N.; Panwar J. Synthesis and Applications of Noble Metal Nanoparticles: A Review. Adv. Sci., Eng. Med. 2017, 9, 527–544. 10.1166/asem.2017.2027. - DOI
-
- Sadykov I. I.; Zabilskiy M.; Clark A. H.; Krumeich F.; Sushkevich V.; van Bokhoven J. A.; Nachtegaal M.; Safonova O. V. Time-Resolved Xas Provides Direct Evidence for Oxygen Activation on Cationic Iron in a Bimetallic Pt-Feo X/Al2o3 Catalyst. ACS Catal. 2021, 11, 11793–11805. 10.1021/acscatal.1c02795. - DOI
-
- Soliman N. Factors Affecting Co Oxidation Reaction over Nanosized Materials: A Review. J. Mater. Res. Technol. 2019, 8, 2395–2407. 10.1016/j.jmrt.2018.12.012. - DOI
LinkOut - more resources
Full Text Sources
